Abstract
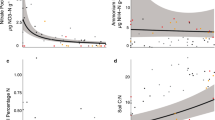
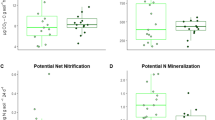
Tree–fungal symbioses are increasingly recognized to affect soil nitrogen (N) transformations, yet the role of free-living soil microbes in the process is largely unclear. Soil microbes directly interact with trees and are a primary driver of many N transformation processes. Here, we explored the linkage among tree mycorrhizal associations, free-living soil microbes and N transformation rates in a temperate forest of Northeast China. Across a gradient of increasing ectomycorrhizal (ECM) tree dominance, we measured soil acid–base chemistry, bacterial and fungal abundances, N-hydrolyzing enzyme activities, abundances and community composition of ammonia-oxidizing archaea (AOA) and bacteria, and net N mineralization and net nitrification rates. Results showed that soil pH, exchangeable base cations, inorganic N concentrations and N transformation rates decreased with increasing ECM tree dominance. The ECM tree dominance was negatively related to soil bacterial and AOA amoA gene abundances, and positively to soil fungal abundances and β-N-acetylglucosaminidase activities. These shifts in soil microbial abundances and enzyme activities along the mycorrhizal gradient were linked with the increase in soil acidity with increasing ECM tree dominance. Piecewise structural equation models revealed that ECM tree dominance was not directly related to N transformation rates, but indirectly to net N mineralization rates by affecting bacterial and fungal abundances, and indirectly to net nitrification rates by influencing AOA amoA gene abundances. Collectively, our results indicate that soil microbes provide a mechanistic link between mycorrhizal associations and soil N transformations, and suggest that shifts in forest mycorrhizal associations under global change could have profound consequences for biogeochemical cycling of temperate forests.







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from corresponding authors on reasonable request.
References
Alves RJE, Wanek W, Zappe A, Richter A, Svenning MM, Schleper C, Urich T (2013) Nitrification rates in Arctic soils are associated with functionally distinct populations of ammonia-oxidizing archaea. ISME J 7:1620–1631
Averill C, Hawkes CV (2016) Ectomycorrhizal fungi slow soil carbon cycling. Ecol Lett 19:937–947
Averill C, Turner BL, Finzi AC (2014) Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505:543–545
Averill C, Dietze MC, Bhatnagar JM (2018) Continental-scale nitrogen pollution is shifting forest mycorrhizal associations and soil carbon stocks. Glob Change Biol 24:4544–4553
Avrahami S, Conrad R (2003) Patterns of community change among ammonia oxidizers in meadow soils upon long-term incubation at different temperatures. App Environ Microbiol 69:6152–6164
Avrahami S, Liesack W, Conrad R (2003) Effects of temperature and fertilizer on activity and community structure of soil ammonia oxidizers. Environ Microbiol 5:691–705
Bahram M, Netherway T, Hildebrand F, Pritsch K, Drenkhan R, Loit K, Anslan S, Bork P, Tedersoo L (2020) Plant nutrient-acquisition strategies drive topsoil microbiome structure and function. New Phytol 227:1189–1199
Bartoń K (2018) MuMIn: Multi-model inference.R package V.1.42.1. https://cran.r-project.org/web/packages/MuMIn/
Bödeker ITM, Clemmensen KE, de Boer W, Martin F, Olson Ã, Lindahl BD (2014) Ectomycorrhizal Cortinarius species participate in enzymatic oxidation of humus in northern forest ecosystems. New Phytol 203:245–256
Bowman WD, Cleveland CC, Halada L, Hreško J, Baron JS (2008) Negative impact of nitrogen deposition on soil buffering capacity. Nat Geosci 1:767–770
Brzostek ER, Greco A, Drake JE, Finzi AC (2013) Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 115:65–76
Cabrera ML, Beare MH (1993) Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci Soc Am J 57:1007–1012
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Carrara JE, Fernandez IJ, Brzostek ER (2021) Mycorrhizal type determines root-microbial responses to nitrogen fertilization and recovery. Biogeochemistry 157(2):245–58. https://doi.org/10.1007/s10533-021-00871-y
Chapman SK, Langley JA, Hart SC, Koch GW (2006) Plants actively control nitrogen cycling: uncorking the microbial bottleneck. New Phytol 169:27–34
Cheeke TE, Phillips RP, Brzostek ER, Rosling A, Bever JD, Fransson P (2017) Dominant mycorrhizal association of trees alters carbon and nutrient cycling by selecting for microbial groups with distinct enzyme function. New Phytol 214:432–442
Cheeke TE, Phillips RP, Kuhn A, Rosling A, Fransson P (2021) Variation in hyphal production rather than turnover regulates standing fungal biomass in temperate hardwood forests. Ecology 102:e03260
Chen D, Lan Z, Bai X, Grace JB, Bai Y (2013) Evidence that acidification-induced declines in plant diversity and productivity are mediated by changes in below-ground communities and soil properties in a semi-arid steppe. J Ecol 101:1322–1334
Cheng L, Booker FL, Tu C, Burkey KO, Zhou LS, Shew HD, Rufty TW, Hu SJ (2012) Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337:1084–1087
Ciccolini V, Bonari E, Ercoli L, Pellegrino E (2016) Phylogenetic and multivariate analyses to determine the effect of agricultural land-use intensification and soil physico-chemical properties on N-cycling microbial communities in drained Mediterranean peaty soils. Biol Fertil Soils 52:811–824
Clarholm M, Skyllberg U (2013) Translocation of metals by trees and fungi regulates pH, soil organic matter turnover and nitrogen availability in acidic forest soils. Soil Biol Biochem 63:142–153
Crowther TW, van den Hoogen J, Wan J, Mayes MA, Keiser AD, Mo L, Averill C, Maynard DS (2019) The global soil community and its influence on biogeochemistry. Science 365:eaav0550
Dick RP (2011) Methods of soil enzymology. Soil Science Society of America, Madison
Fernandez CW, Kennedy PG (2016) Revisiting the ‘Gadgil effect’: do interguild fungal interactions control carbon cycling in forest soils? New Phytol 209:1382–1394
Fierer N, Jackson JA, Vilgalys R, Jackson RB (2005) Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol 71:4117–4120
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci USA 102:14683–14688
Gubry-Rangin C, Kratsch C, Williams TA, McHardy AC, Embley M, Prosser JI, Macqueen DJ (2015) Coupling of diversification and pH adaptation during the evolution of terrestrial Thaumarchaeota. Proc Natl Acad Sci USA 112:9370–9375
Heděnec P, Nilsson LO, Zheng HF, Gundersen P, Schmidt IK, Rousk J, Vesterdal L (2020) Mycorrhizal association of common European tree species shapes biomass and metabolic activity of bacterial and fungal communities in soil. Soil Biol Biochem 149:107933
Hobbie SE (1992) Effects of plant species on nutrient cycling. Trends Ecol Evol 7:336–339
Högberg MN, Chen Y, Högberg P (2007) Gross nitrogen mineralisation and fungi-to-bacteria ratios are negatively correlated in boreal forests. Biol Fertil Soils 44:363–366
Isobe K, Ise Y, Kato H, Oda T, Vincenot CE, Koba K, Tateno R, Senoo K, Ohte N (2020) Consequences of microbial diversity in forest nitrogen cycling: diverse ammonifiers and specialized ammonia oxidizers. ISME J 14:12–25
Jilling A, Keiluweit M, Contosta AR, Frey S, Schimel J, Schnecker J, Smith RG, Tiemann L, Grandy AS (2018) Minerals in the rhizosphere: overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry 139:103–122
Jo I, Fei S, Oswalt CM, Domke CM, Phillips RP (2019) Shifts in dominate tree mycorrhizal associations in response to anthropogenic impacts. Sci Adv 5:eaav6358
Keller AB, Phillips RP (2019) Leaf litter decay rates differ between mycorrhizal groups in temperate, but not tropical, forests. New Phytol 222:556–564
Kieloaho A, Pihlatie M, Carrasco MD, Kanerva S, Parshintsev J, Riekkola M, Pumpanen J, Heinonsalo J (2016) Stimulation of soil organic nitrogen pool: the effect of plant and soil organic matter degrading enzymes. Soil Biol Biochem 96:97–106
Kits KD, Sedlacek CJ, Lebedeva EV, Han P, Bulaev A, Pjevac P, Daebeler A, Romano S, Albertsen M, Stein LY, Daims H, Wagner M (2017) Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature 549:269–272
Knops JMH, Bradley KL, Wedin DA (2002) Mechanisms of plant species impacts on ecosystem nitrogen cycling. Ecol Lett 5:454–466
Kohler A, Kuo A, Nagy LG, Morin E, Barry KW et al (2015) Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat Genet 47:410–415
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Evol Genet Anal 33:1870–1874
Kuypers MM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nat Rev Microbiol 16:263–276
Lefcheck JS (2016) PIECEWISESEM: piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol Evol 7:573–579
Lin GG, McCormack ML, Ma CE, Guo DL (2017) Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytol 213:1440–1451
Lin GG, Guo DL, Li L, Ma CE, Zeng DH (2018) Contrasting effects of ectomycorrhizal and arbuscular mycorrhizal tropical tree species on soil nitrogen cycling: the potential mechanisms and corresponding adaptive strategies. Oikos 127:518–530
Lin YX, Ye GP, Luo J, Di H, Liu DY, Fan JB, Ding WX (2019) Nitrosospira cluster 8a plays a predominant role in the nitrification process of a subtropical Ultisol under long-term inorganic and organic fertilization. Appl Environ Microbiol 84:e01031
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963
Mann PJ, Sobczak W, LaRue MM, Bulygina E, Davydova A, Vonk J, Schade J, Davydov S, Zimov N, Holmes RM, Spencer RGM (2014) Evidence for key enzymatic controls on metabolism of Arctic river organic matter. Glob Change Biol 20:1089–1100
McDowell NG, Allen CD, Anderson-Teixeira K, Aukema BH, Bond-Lamberty B et al (2020) Pervasive shifts in forest dynamics in a changing world. Science 368:eaaz9463
Midgley MG, Phillips RP (2016) Resource stoichiometry and the biogeochemical consequences of nitrogen deposition in a mixed deciduous forest. Ecology 97:3369–3378
Mushinski RM, Phillips RP, Payne ZC, Abney RB, Jo I, Fei SL, Pusede SE, White JR, Rusch DB, Raff JD (2019) Microbial mechanisms and ecosystem flux estimation for aerobic NOy emissions from deciduous forest soils. Proc Natl Acad Sci USA 116:2138–2145
Mushinski RM, Payne ZC, Raff JD, Craig ME, Pusede SE, Rusch DB, White JR, Phillips RP (2021) Nitrogen cycling microbiomes are structured by plant mycorrhizal associations with consequences for nitrogen oxide fluxes in forests. Glob Change Biol 27:1068–1082
Norman JS, Barrett JE (2016) Substrate availability drives spatial patterns in richness of ammonia-oxidizing bacteria and archaea in temperate forest soils. Soil Biol Biochem 94:169–172
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P et al (2019) VEGAN: Community ecology package. R package V.2.5-7. https://cran.r-project.org/web/packages/vegan/
Op De Beeck M, Troein C, Peterson C, Persson P, Tunlid A (2018) Fenton reaction facilitates organic nitrogen acquisition by an ectomycorrhizal fungus. New Phytol 218:335–343
Osburn ED, Barrett JE (2020) Abundance and functional importance of complete ammonia-oxidizing bacteria (comammox) versus canonical nitrifiers in temperate forest soils. Soil Biol Biochem 145:107801
Parham JA, Deng SP (2000) Detection, quantification and characterization of β-glucosaminidase activity in soil. Soil Biol Biochem 32:1183–1190
Petersen DG, Blazewicz SJ, Firestone M, Herman DJ, Turetsky M, Waldrop M (2012) Abundance of microbial genes associated with nitrogen cycling as indices of biogeochemical process rates across a vegetation gradient in Alaska. Environ Microbiol 14:993–1008
Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon–nutrient couplings in temperate forests. New Phytol 199:41–51
Pinheiro J, Bates D, DebRoy S, Sarkar D (2019) NLME: Linear and nonlinear mixed effects models. R package V.3.1–143. https://cran.r-project.org/package=nlme/
Prosser JI, Nicol GW (2012) Archaeal and bacterial ammonia-oxidisers in soil: the quest for niche specialisation and differentiation. Trends Microbiol 20:523–531
Prosser JI, Hink L, Gubry-Rangin C, Nicol GW (2020) Nitrous oxide production by ammonia oxidizers: physiological diversity, niche differentiation and potential mitigation strategies. Glob Change Biol 26:103–118
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Rousk J, Brookes PC, Bååth E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microbiol 75:1589–1596
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
See CR, McCormack ML, Hobbie SE, Flores-Moreno H, Silver WL, Kennedy PG (2019) Global patterns in fine root decomposition: climate, chemistry, mycorrhizal association and woodiness. Ecol Lett 22:946–953
Shi XZ, Hu HW, Wang JQ, He JZ, Zheng CY, Wan XH, Huang ZQ (2018) Niche separation of comammox Nitrospira and canonical ammonia oxidizers in an acidic subtropical forest soil under long-term nitrogen deposition. Soil Biol Biochem 126:114–122
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD et al (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Soudzilovskaia NA, Vaessen S, Barcelo M, He JH, Rahimlou S, Abarenkov K, Brundrett MC, Gomes SIF, Merckx V, Tedersoo L (2020) FungalRoot: global online database of plant mycorrhizal associations. New Phytol 227:955–966
Tatsumi C, Taniguchi T, Du S, Yamanaka N, Tateno R (2020) Soil nitrogen cycling is determined by the competition between mycorrhiza and ammonia-oxidizing prokaryotes. Ecology 101:e02963
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycling: sources and consequences. Ecol Appl 7:737–750
Wang XG, Wiegand T, Hao ZQ, Li BH, Ye J, Lin F (2010) Species associations in an old-growth temperate forest in north-eastern China. J Ecol 98:674–686
Waring BG, Averill C, Hawkes C (2013) Differences in fungal and bacterial physiology alter soil carbon and nitrogen cycling: insights from meta-analysis and theoretical models. Ecol Lett 16:887–894
Weemstra M, Peay KG, Davies SJ, Mohamad M, Itoh A, Tan S, Russo SE (2020) Lithological constraints on resource economies shape the mycorrhizal composition of a Bornean rain forest. New Phytol 228:253–268
Wurzburger N, Brookshire ENJ, McCormack ML, Lankau RA (2017) Mycorrhizal fungi as drivers and modulators of terrestrial ecosystem processes. New Phytol 213:996–999
Yao HY, Campbell CD, Chapman SJ, Freitag TE, Nicol GW, Singh BK (2013) Multi-factorial drivers of ammonia oxidizer communities: evidence from a national soil survey. Environ Microbiol 15:2545–2556
Yin HJ, Wheeler E, Phillips RP (2014) Root-induced changes in nutrient cycling in forests depend on exudation rates. Soil Biol Biochem 78:213–221
Yuan ZQ, Wang SP, Gazol A, Mellard J, Lin F, Ye J, Hao ZQ, Wang XG, Loreau M (2016) Multiple metrics of diversity have different effects on temperate forest functioning over succession. Oecologia 182:1175–1185
Zhu B, Gutknecht J, Herman DJ, Keck DC, Firestone MK, Cheng WX (2014) Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol Biochem 76:183–192
Acknowledgements
We are grateful to the National Research Station of Changbai Mountain Forest Ecosystems for providing the experimental site and relevant supports. We thank Dr. Stephen Sebestyen and two anonymous reviewers for suggestions that greatly improve our manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 31700538 and 31830015) and the Youth Innovation Promotion Association CAS (No. 2019200).
Author information
Authors and Affiliations
Contributions
GL, DZ, XW conceived the study; GL, ZY and YZ collected and analyzed the data; All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, G., Yuan, Z., Zhang, Y. et al. Dominant tree mycorrhizal associations affect soil nitrogen transformation rates by mediating microbial abundances in a temperate forest. Biogeochemistry 158, 405–421 (2022). https://doi.org/10.1007/s10533-022-00909-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-022-00909-9