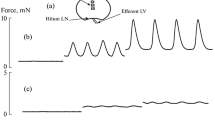
Inflammation accompanies most pathological processes, while the lymphatic system takes part in both the development and resolution of inflammation. We studied the contractile function of rat lymph nodes after cecal ligation and puncture (CLP). In 24 h after CLP, the mesenteric lymph nodes were removed and placed in the myograph chamber. After CLP, the lymph nodes showed lower tension than lymph nodes from sham-operated animals (control). The expression of inducible NO synthase, cyclooxygenase-2, and cystathionine-γ-lyase was observed in the lymph nodes of CLP rats. NO, prostaglandins, and H2S formed during inflammation inhibited contractile activity of smooth muscle cells in the capsule of the lymph nodes, which manifested itself in inhibition of phase contractions and a decrease in the tone of their capsule.
Similar content being viewed by others
References
Breslin JW, Yang Y, Scallan JP, Sweat RS, Adderley SP, Murfee WL. Lymphatic vessel network structure and physiology. Compr. Physiol. 2018;9(1):207-299. https://doi.org/10.1002/cphy.c180015
Lobov GI, Pan’kova MN. Heparin inhibits contraction of smooth muscle cells in lymphatic vessels. Bull. Exp. Biol. Med. 2010;149(1):4-6. https://doi.org/10.1007/s10517-010-0860-0
Liao S, von der Weid PY. Lymphatic system: an active pathway for immune protection. Semin. Cell Dev. Biol. 2015;38:83-89. https://doi.org/10.1016/j.semcdb.2014.11.012
Ohtani O, Ohtani Y. Structure and function of rat lymph nodes. Arch. Histol. Cytol. 2008;71(2):69-76. https://doi.org/10.1679/aohc.71.69
Cleypool CGJ, Mackaaij C, Lotgerink Bruinenberg D, Schurink B, Bleys RLAW. Sympathetic nerve distribution in human lymph nodes. J. Anat. 2021;239(2):282-289. https://doi.org/10.1111/joa.13422
Lobov GI, Pan’kova MN. Lymph flow: role of lymph nodes. Regionar. Krovoobr. Mikrotsirk. 2012;11(2):52-56. Russian. https://doi.org/10.24884/1682-6655-2012-11-2-52-56
Arasa J, Collado-Diaz V, Kritikos I, Medina-Sanchez JD, Friess MC, Sigmund EC, Schineis P, Hunter MC, Tacconi C, Paterson N, Nagasawa T, Kiefer F, Makinen T, Detmar M, Moser M, Lämmermann T, Halin C. Upregulation of VCAM-1 in lymphatic collectors supports dendritic cell entry and rapid migration to lymph nodes in inflammation. J. Exp. Med. 2021;218(7):e20201413. https://doi.org/10.1084/jem.20201413
Garcia LF, Singh V, Mireles B, Dwivedi AK, Walker WE. Common Variables That Influence Sepsis Mortality in Mice. J. Inflamm. Res. 2023;16:1121-1134. https://doi.org/10.2147/JIR.S400115
Bouta EM, Wood RW, Perry SW, Brown EB, Ritchlin CT, **ng L, Schwarz EM. Measuring intranodal pressure and lymph viscosity to elucidate mechanisms of arthritic flare and therapeutic outcomes. Ann. NY Acad. Sci. 2011;1240:47-52. https://doi.org/10.1111/j.1749-6632.2011.06237.x
Zhang N, Deng J, Wu F, Lu X, Huang L, Zhao M. Expression of arginase I and inducible nitric oxide synthase in the peripheral blood and lymph nodes of HIV-positive patients. Mol. Med. Rep. 2016;13(1):731-743. https://doi.org/10.3892/mmr.2015.4601
Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31(5):986-1000. https://doi.org/10.1161/ATVBAHA.110.207449
Dunn WR, Alexander SP, Ralevic V, Roberts RE. Effects of hydrogen sulphide in smooth muscle. Pharmacol. Ther. 2016;158:101-113. https://doi.org/10.1016/j.pharmthera.2015.12.007
Lo Faro ML, Fox B, Whatmore JL, Winyard PG, Whiteman M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide. 2014;41:38-47. https://doi.org/10.1016/j.niox.2014.05.014
Tripatara P, Patel NS, Collino M, Gallicchio M, Kieswich J, Castiglia S, Benetti E, Stewart KN, Brown PA, Yaqoob MM, Fantozzi R, Thiemermann C. Generation of endogenous hydrogen sulfide by cystathionine gamma-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab. Invest. 2008;88(10):1038-1048. https://doi.org/10.1038/labinvest.2008.73
Miller TW, Wang EA, Gould S, Stein EV, Kaur S, Lim L, Amarnath S, Fowler DH, Roberts DD. Hydrogen sulfide is an endogenous potentiator of T cell activation. J. Biol. Chem. 2012;287(6):4211-4221. https://doi.org/10.1074/jbc.M111.307819
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 176, No. 9, pp. 280-285, September, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kosareva, M.E., Chivildeev, A.V. & Lobov, G.I. Sepsis-Induced Inhibition of Contractile Function of Lymphatic Nodes. Bull Exp Biol Med 176, 305–309 (2024). https://doi.org/10.1007/s10517-024-06013-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-024-06013-2