Abstract
Ticks require blood feeding on vertebrate animals throughout their life cycle, and also concentrate the iron-containing blood, resulting in a high concentration of hydrogen peroxide (H2O2). High concentrations of H2O2 are harmful to organisms, due to their serious damage of macromolecules. Ticks have antioxidant enzymes, such as peroxiredoxins (Prxs), that scavenge H2O2. Prxs may have important roles in regulating the H2O2 concentration in ticks during blood feeding and oviposition. Moreover, Prxs are considered potential vaccine candidates in other parasites, such as Leishmania and Fasciola. In the present study, the efficacy of a tick Prx (HlPrx2) as a vaccine candidate antigen was evaluated. First, recombinant HlPrx2 (rHlPrx2) was expressed in Escherichia coli, and then, its purity and endotoxin levels were confirmed prior to administration. The rHlPrx2 proteins were of high purity with acceptably low endotoxin levels. Second, the ability of rHlPrx2 administration to stimulate mouse immunity was evaluated. The rHlPrx2 protein, with or without an adjuvant, could stimulate immunity in mice, especially the IgG1 of Th2 immune response. Using Western blot analysis, we also observed whether rHlPrx2-immunized mice sera could recognize native HlPrx2 protein in crude tick midgut proteins. Western blot analysis demonstrated that rHlPrx2-administrated mouse sera could detect the native HlPrx2. Finally, the effects of rHlPrx2 immunization in mice were studied using nymphal ticks. Although the challenged ticks were not affected by rHlPrx2 immunization, rHlPrx2 still might be considered as a vaccine candidate against ticks because of its high immunogenicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks are obligate hematophagous arthropods. They require blood feeding throughout their life cycle. Blood feeding and blood digesting provide nutrition and energy for development, molting, and embryogenesis in ticks (Grandjean 1983). Ticks feed on vertebrate blood containing high levels of free iron, such as heme and ferrous iron. Ticks also concentrate host blood with iron; this concentration of the blood leads to high levels of iron in ticks. Host-derived iron may react with oxygen in the tick body, resulting to high concentrations of reactive oxygen species (ROS), including hydrogen peroxide (H2O2) (Citelli et al. 2007; Galay et al. 2015). H2O2 in high concentrations is known to be harmful to aerobic organisms due to its ability to seriously damage membrane lipids, nucleic acids, and proteins (Robinson et al. 2010).
To scavenge H2O2, ticks have antioxidant enzymes, such as catalases (Kumar et al. 2016) and peroxiredoxins (Prxs) (Tsuji et al. 2001; Kusakisako et al. 2016a). While gene silencing of Prxs in ticks can affect ticks’ blood feeding and oviposition (Kusakisako et al. 2016b), gene silencing of catalase in ticks has no significant effect (Kumar et al. 2016). These results indicate that Prxs may have an important role in tick blood feeding and oviposition through the regulation of the H2O2 concentration.
Likewise, Prxs are considered to be vaccine candidates against Leishmania donovani (Daifalla et al. 2011) or Fasciola hepatica (Raina et al. 2011). At the infective stage, F. hepatica flukes excyst from a dormant state following ingestion and penetrate the intestinal wall before migrating to the liver; in this nutrient- and oxygen-rich environment, the flukes undergo rapid growth and development, and energy is supplied by aerobic respiration (Sekiya et al. 2006). The developmental situation of Fasciola parasites is similar to the development of ticks during blood feeding. In addition, Fasciola parasites secrete Prxs into their host to regulate their environment for survival in the host body (Dalton et al. 2013). These results strongly suggest that tick Prxs could help ticks’ successful blood feeding in a way similar to that of Fasciola parasites. Therefore, we consider tick Prxs to be a potential target for tick control that could provide further understanding of oxidative stress co** mechanisms in ticks during blood feeding. In the present study, we evaluated the efficacy of Haemaphysalis longicornis 2-Cys peroxiredoxin (HlPrx2) as an antigen candidate for the vaccine.
Materials and methods
Ticks and animals
A parthenogenetic H. longicornis population (Okayama strain) (Fujisaki 1978) was maintained for several generations by feeding on the ears of Japanese white rabbits (Kyudo, Saga, Japan) at the Experimental Animal Center, Joint Faculty of Veterinary Medicine, Kagoshima University, Kagoshima, Japan. Four-week-old female BALB/c mice (BALB/cN Sea, Kyudo) were used for vaccination. Animals in our experiments were used in accordance with approved guidelines (approval numbers VM15055 and VM15056) from the Animal Care and Use Committee of Kagoshima University.
Expression and purification of recombinant Haemaphysalis longicornis 2-Cys peroxiredoxin (HlPrx2)
Recombinant HlPrx2 (rHlPrx2) was expressed as a histidine (His)-tagged protein using the expression vector pRSET C (Invitrogen, Carlsbad, CA, USA) as described previously (Kusakisako et al. 2016a). Briefly, the open reading frame sequence was amplified using polymerase chain reaction (PCR). The amplified PCR product was then purified using a GENECLEAN® II KIT (MP Biomedical, Solon, OH, USA) and subcloned into the frame of the pRSET C. rHlPrx2 was expressed in Escherichia coli BL21 (DE3) strain and purified using the Biologic DuoFlow™ Chromatography System (Bio-Rad, Hercules, CA, USA) with a HisTrap FF column (GE Healthcare, Pittsburgh, PA, USA); the collected fractions were then dialyzed using a dialysis tube against phosphate buffered saline (PBS) at 4 °C. Finally, the resulting rHlPrx2 protein was adjusted to a concentration of 1 mg/ml.
Protein and endotoxin analysis
The E. coli lysate and purified rHlPrx2 proteins were resolved in a 12% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. After SDS-PAGE, the gel was stained by Coomassie Brilliant Blue (CBB) and viewed using Gel Doc (Bio-Rad). Endotoxin measurement was performed using a ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit (GenScript, Piscataway, NJ, USA).
Immunization protocol of mice
At 3-week intervals, female 4-week-old BALB/c mice (Kyudo) were twice immunized subcutaneously with 30 µg of rHlPrx2 alone or rHlPrx2 mixed with an equal volume of Incomplete Freund’s Adjuvant (IFA). Control mice were injected with PBS or PBS + IFA. Six mice for each group were used in this study. Antisera were collected from the orbital sinus under anesthesia before the first administration and 1 week after each administration (1st administration = administration; 2nd administration = booster). Antigen-specific antibody titers were evaluated by enzyme-linked immunosorbent assay (ELISA).
Immune response
The specific antibody titers of the immunized mice were determined by ELISA. Ninety-six-well Nunc MaxiSorp ELISA plates (Nunc, Roskilde, Denmark) were coated with rHlPrx2 diluted in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) (100 ng in 100 μl/well) overnight at 4 °C. The plates were washed three times with 0.05% PBS-Tween 20 (PBS-T), and the wells were saturated with 150 μl of 1% skim milk in PBS (saturation solution) for 1 h at room temperature (RT). The saturation solution was discarded, and the plates were washed four times with PBS-T. The wells were incubated for 1 h at RT in the presence of 100 μl of five-time serial dilutions of tested mice sera in a saturation solution. The initial dilution of total IgG and IgG1 began at 1:100, while that of IgG2a started at 1:50. After washing the plates six times with PBS-T, the wells were incubated with 100 μl of different secondary antibody solutions for 1 h at RT. To detect specific total IgG, IgG1, and IgG2a, the following antibodies diluted in a saturation solution were used:
-
IgG—Horseradish peroxidase (HRP)-conjugated Goat Anti-Mouse IgG (1:4000—SouthernBiotech, Birmingham, AL, USA)
-
IgG1—HRP-conjugated Goat Anti-Mouse IgG1 (1:4000—SouthernBiotech)
-
IgG2a—HRP-conjugated Goat Anti-Mouse IgG2a (1:4000—SouthernBiotech)
The plates were again washed six times with PBS-T, and SureBlue™ TMB Microwell peroxidase substrate (KPL, Gaithersburg, MD, USA) was added at 100 μl/well and incubated at 37 °C for 30 min. The reaction was stopped by the addition of 50 μl of stop solution (equal volumes of 0.5 N HCl and 0.3 N sulfuric acid mixed). The absorbance at 450 nm was read in a Bio-Rad Novapath ELISA plate reader (Bio-Rad). Antibody titers were defined as the dilution rate (log10) under 0.5 < OD450 nm.
Protein extraction and Western blot analysis
Homogenized partially fed tick midguts were suspended in PBS and sonicated for six min at 45 kHz using a VS-100III ultrasonic cleaner (AS ONE Corporation, Osaka, Japan) and then centrifuged at 500×g. The supernatant was resolved in SDS-PAGE gel under reducing conditions. After SDS-PAGE, the proteins were transferred onto a polyvinylidene difluoride membrane (Immobilon®-P; Millipore, Danvers, MA, USA). The membranes were blocked for 1 h at RT with 0.3% skim milk in PBS-T (blocking solution); they were incubated with 1:100 dilutions of rHlPrx2-immunized mouse sera (sera containing the highest titers during tick infestation were used in each immunized group) and a 1:500 dilution of anti-rHlPrx2 mouse sera for positive control (Kusakisako et al. 2016b) in a blocking solution at 4 °C overnight. For loading control, tubulin was detected using antiserum against recombinant H. longicornis tubulin (Umemiya-Shirafuji et al. 2012). After washing three times in PBS-T, the membranes were incubated with a 1:50,000 dilution of HRP-conjugated sheep anti-mouse IgG (Dako, Glostrup, Denmark) in a blocking solution at RT for 1 h. After washing three times in PBS-T, bands were detected using Clarity™ Western ECL Substrate (Bio-Rad) and viewed using FluorChem®FC2 software (Alpha Innotech, San Leandro, CA, USA).
Tick infestation on rHlPrx2-vaccinated mice
To evaluate the effects of the rHlPrx2 immunization of mice against ticks, 20 nymphal H. longicornis ticks were allowed to feed on an immunized mouse, 3 weeks after the booster, until fully engorged by the feeding capsule method (Anisuzzamman et al. 2012). The total number of ticks that successfully engorged divided by the number of infested ticks (engorged rate), the total number of nymphal ticks molted to adult ticks divided by the number of successfully engorged ticks (molting rate), and the survival rate after molting from nymphs to adult ticks were assessed. Mouse antisera were collected by exsanguination under anesthesia after tick infestations were finished. Antigen-specific antibody titers were also evaluated by ELISA, using the protocols mentioned above.
Statistical analysis
A one-way ANOVA test was applied to the obtained data, and statistically significant differences (P < 0.001) in the antibody titers of the same state’s or group’s antisera were demonstrated. For pair comparisons within groups, Tukey’s test was applied. Data, from tick infestations on the vaccinated mice, except for the engorged body weight, were statistically analyzed using a Chi square test. Analysis of the engorged body weight was done using Welch’s t test.
Results
rHlPrx2 protein expression and purification
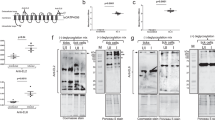
rHlPrx2 proteins were expressed in E. coli as inclusion bodies and purified using the Biologic DuoFlow™ Chromatography System. The E. coli lysate and purified rHlPrx2 proteins were analyzed in SDS-PAGE. SDS-PAGE demonstrated a single band with a molecular mass of approximately 27 kDa including His-tag (Fig. 1, arrowhead). The endotoxin levels of the final purified samples were less than 0.02 endotoxin units (EU) per 30 µg of purified rHlPrx2, which were used for vaccinating mice (Table 1). In addition, Brito and Singh (2011) stated that endotoxin levels in the vaccine type of recombinant subunit for preclinical research of less than 20 EU/ml are acceptable. These results suggest that the rHlPrx2 was pure and has an acceptable endotoxin level for vaccinating mice.
Purification of recombinant Haemaphysalis longicornis 2-Cys peroxiredoxin (rHlPrx2). rHlPrx2 was expressed in E. coli and purified by fast protein liquid chromatography (FPLC). The E. coli lysate and purified rHlPrx2 were analyzed by SDS-PAGE and CBB staining. The molecular weight is shown on the left side of the image. The arrowhead indicates a band of rHlPrx2 protein
Antibody titers after immunization
Titers of rHlPrx2 with or without an IFA group (rHlPrx2 + IFA or rHlPrx2) using total IgG secondary antibodies significantly increased after the administration and booster or the administration, respectively. However, the titer post tick infestation was almost the same as the booster (Fig. 2A, rHlPrx2 and rHlPrx2 + IFA). The total IgG antibody titers of the rHlPrx2 and rHlPrx2 + IFA groups were significantly higher than those of the control groups (PBS and PBS + IFA) after the administration, booster, and tick infestation. In addition, the total IgG antibody titers of the rHlPrx2 + IFA group showed higher titers as compared with the rHlPrx2 group in all states (Fig. 2A). Using the IgG1 isotype as a marker for Th2 lymphocytes, the antibody titers showed almost the same transition with the total IgG transition (Fig. 2B). Using the IgG2a isotype as a marker for Th1 lymphocytes, although the antibody titers of rHlPrx2 + IFA group significantly increased after the administration and booster, the titer post tick infestation had almost the same levels as post booster (Fig. 2C, rHlPrx2 + IFA). Interestingly, the titers of the rHlPrx2 group increased slightly after the booster but had almost the same titer levels post tick infestation (Fig. 2C, rHlPrx2). The IgG2a antibody titers of the rHlPrx2 + IFA group were significantly higher than those of the control groups after administration, booster, and tick infestation. On the other hand, the IgG2a antibody titers of the rHlPrx2 group showed significantly, but slightly higher titers as compared with those of control groups post booster and tick infestation (Fig. 2C).
The trend of the antibody titer in immunized mouse sera. Antibody titers were defined as the dilution rate (log10) under 0.5 < OD450 nm. Pre-Adm., Pre-Administration; Adm., 1st administration after 1 week; Booster, 2nd administration after 1 week; Tick Inf., after Tick infestation; aP < 0.01, significant differences versus PBS and PBS + IFA groups; bP < 0.01, significant differences between rHlPrx2 + IFA and rHlPrx2 groups; cP < 0.05, significant differences versus PBS and PBS + IFA groups; *P < 0.05, significant differences between the states of immunization; **P < 0.01, significant differences between the states of immunization. PBS phosphate buffered saline; IFA Incomplete Freund’s Adjuvant; rHlPrx2 recombinant Haemaphysalis longicornis 2-Cys peroxiredoxin
IgG1 titers were higher than IgG2a titers (Fig. 2B, C). In addition, the total IgG and IgG1 antibody titers showed almost the same trend in the rHlPrx2 group (Fig. 2A, B). These results suggest that rHlPrx2 could induce especially a Th2—but not a Th1—immune response. Moreover, vaccination using rHlPrx2 alone could induce high antibody titers of total IgG and IgG1 titers as compared with those of control groups, indicating that the rHlPrx2 protein could be highly immunogenic to the host.
The recognition of native HlPrx2 protein in the crude tick protein sample using immunized mouse sera
We also evaluated whether rHlPrx2-immunized mouse sera could recognize native HlPrx2 protein from a crude tick protein sample using Western blot analysis. The post tick infestation state in each group showed the highest antibody titers. For this reason, we used those sera from the same mouse as the first antibody in each immunization state. The partially fed tick midgut proteins were used as crude tick protein samples, since the native HlPrx2 proteins were significantly upregulated in the midgut during the partially fed stage of tick blood feeding, and the HlPrx2 protein expression levels had almost the same level and trend in nymphs and adults (Kusakisako et al. 2016b). In the rHlPrx2 + IFA immunized group, the native HlPrx2 protein could be detected after the administration of the rHlPrx2 protein (Fig. 3, rHlPrx2 + IFA). In addition, the rHlPrx2-immunized group’s sera could detect the native HlPrx2 protein after the booster (Fig. 3, rHlPrx2). The control immunized groups’ sera could not detect the native HlPrx2 protein (Fig. 3, PBS and PBS + IFA). These results suggest that antibodies in the mice induced by rHlPrx2 protein immunization can bind to recognize the native HlPrx2 protein; therefore, the rHlPrx2 proteins used could have retained almost the same structure of the native HlPrx2 protein in mice.
Detection of native HlPrx2 protein from partially fed ticks’ midguts using rHlPrx2-immunized mouse sera. The left column indicates the molecular weight markers at 30 and 20.1 kDa. Tick tubulin protein served as the control. The right side indicates different stages of immunization. The serum from the different stages of immunization was from the same mouse. The anti-rHlPrx2 serum used for a positive control (PC) was from the previous study (Kusakisako et al. 2016b). The arrowheads indicate the bands of native HlPrx2 protein having a molecular mass of approximately 23 kDa. PBS phosphate buffered saline; IFA Incomplete Freund’s Adjuvant; rHlPrx2 recombinant Haemaphysalis longicornis 2-Cys peroxiredoxin; PC positive control
Effects of immunization on Haemaphysalis longicornis nymphal ticks
After immunization twice with rHlPrx2, mice were challenged with nymphal ticks. Twenty ticks were infested on each mouse and allowed to feed until engorgement. However, there were no significant differences in the engorged rate, engorged body weight, molting rate, and survival rate after molting among ticks detached from vaccinated mice (Table 2). These results suggest that immunization with rHlPrx2 had little or no effect on the blood feeding of nymphal ticks.
Discussion
In endoparasites, Prxs have been shown to be the most important detoxifying enzyme for their survival (Kawazu et al. 2008; Dalton et al. 2013), making it a candidate for use in vaccine development and as a therapeutic target in treating endoparasitic infectious diseases (Rudenko et al. 2005; Peterson and Luckhart 2006). Studies using Prxs as vaccines have been performed with L. donovani (Daifalla et al. 2011) and F. hepatica (Donnelly et al. 2008; Mendes et al. 2010; Raina et al. 2011). Although there have not been studies reporting on tick Prx vaccination and host immune responses against tick Prxs, H. longicornis 1-Cys Prx protein (HlPrx), in the same family as HlPrx2, was detected in host sera infested with several ticks over a short interval (Tsuji et al. 2001). These results suggest that tick Prxs may be secreted into their hosts and could be a candidate for both vaccine and immunotherapeutic development. Therefore, we performed the vaccine experiment in mice using the recombinant tick Prx protein (HlPrx2) in the present study.
Prxs have been studied for their role in parasite survival and virulence necessitating the production of efficient defenses against ROS by the host immune system (Sekiya et al. 2006; Gretes et al. 2012; Dalton et al. 2013). In addition, L. donovani Prx is an antigen that elicited a high level of IgG1 as a marker of Th2 lymphocytes, but not IgG2a as a marker of Th1 lymphocytes (Daifalla et al. 2011). In the present study, we evaluated the vaccine efficacy of HlPrx2 in mice using recombinant HlPrx2 with or without IFA. The results demonstrated that the vaccine with or without IFA led to almost the same antibody titer against total IgG and IgG1 as a marker of Th2 lymphocytes. On the other hand, IgG2a as a marker for Th1 lymphocytes was low titer in the rHlPrx2 without IFA vaccinated groups. These phenomena suggest that the induction of the host’s Th2 immune response could lead to low levels of the host Th1 immune response; thus, the parasites could escape from the host’s Th1 immune response through, for instance, migrations of macrophages, dendritic cells, and neutrophils to eliminate the parasites.
We also evaluated the vaccine efficacy for the challenge of nymphal ticks to the immunized mice. However, the effects of HlPrx2 vaccination against ticks’ engorgement success rate, engorged body weight, molting rate to adult stage, and survival rate after molting were small or nonexistent. There have been a few reports regarding tick Prxs. Anti-HlPrx antibodies were detected in the host serum after several repeated tick infestations (Tsuji et al. 2001), suggesting that tick Prxs might be released from ticks into the host and that the amount of released HlPrx protein was quite small, since several infestations of ticks were needed for the anti-HlPrx antibody to be successfully detected. In addition, another report (Daifalla et al. 2011) and the present study (Fig. 2) have demonstrated that Prxs without the adjuvant could induce high antibody titers, especially in Th2 immune responses, such as that of IgG1. The IgG1 antibody response is promoted by the Th2 immune response, which is counterbalanced by a Th1 immune response (Yanase et al. 2014). In the tick challenge experiment, the induction of the Th2 immune response in mice by rHlPrx2 immunization and tick infestations could lead to low levels of the host Th1 immune response; therefore, ticks would escape host Th1 immune responses. These results demonstrated that immunization with the rHlPrx2 protein would have little to no effect on nymphal ticks during and after blood feeding. On the other hand, there are two strains of H. longicornis ticks, the parthenogenetic strain and bisexual strain. Although the HlPrx2 gene was present in the bisexual strain and the genome sequence was slightly different between the two strains, the amino acid sequence of HlPrx2 in the bisexual strain was completely the same with the amino acid sequence of HlPrx2 in the parthenogenetic strain (data not shown). However, the bisexual strain has male and female adult stages, thus, there might be some sex related responses against the vaccination of HlPrx2 to their hosts. To avoid such case, we decided to use the parthenogenetic strain of H. longicornis tick.
Although the effects of rHlPrx2 immunization had little to no effect, we demonstrated that immunization with the rHlPrx2 protein could only induce high and acceptable antibody titers, even as compared with the immunization with the rHlPrx2 protein with IFA. In addition, we had conducted a similar experiment using rabbits. When the rabbits were vaccinated with the rHlPrx2, high antibody titer against the rHlPrx2 protein can be induced. Moreover, the effect of specific antibody against rHlPrx2 in the rabbit was also evaluated by tick infestation using the parthenogenetic adult H. longicornis ticks; however, the rHlPrx2 vaccination on rabbits did not significantly affect the ticks’ engorgement, engorged body weight, egg weight, and hatching rate. It is also notable that rHlPrx2 vaccination on rabbits without IFA slightly depresses the ticks’ engorged body weight and egg weight (Tanaka et al. 2016). These phenomena were considered to be the reason that the reduced 2-Cys Prxs are typically in the form of decamers arranged in a ring-like toroid structure. During peroxidatic cycling, decamers dissociate into dimers upon disulfide formation and are regained upon disulfide reduction (Wood et al. 2002; Hall et al. 2011). These results suggest that 2-Cys Prxs without the adjuvant could induce high antibody titers because 2-Cys Prxs can form multimers. Actually, the rHlPrx2 proteins form an oligomer (Kusakisako et al. 2016a). On the other hand, immunization with rHlPrx2 proteins especially stimulated IgG1 antibodies related to Th2 immune responses (Fig. 2). In helminth parasites, helminth infection induces M2 macrophages, related to Th2 immune responses, to the site of the infection, and the helminth recombinant Prx, which is inoculated intraperitoneally to mice, also induces M2 macrophages (Donnelly et al. 2008). Moreover, M2 macrophages can promote differentiation from Th0 to Th2 lymphocytes (Satoh et al. 2010). Thus, rHlPrx2 proteins might induce M2 macrophages and stimulate the host Th2 lymphocytes in vaccinated mice.
In animal models challenged with pathogens such as Coccidioides immitis (Abuodeh et al. 1999), Listeria monocytogenes (Miller et al. 1995), Schistosoma mansoni (Mountford et al. 1996), respiratory syncytial virus (Li et al. 1998), and Candida albicans (Cárdenas-Freytag et al. 1999), vaccines inducing Th1 immune responses have been proven highly effective at preventing infections, whereas vaccines inducing Th2 immune responses increase sensitivity to infection. Therefore, although Th1 immune responses are key to protecting against most infections, the vaccines and passive immunization rely on Th2 immune responses (Spellberg and Edwards 2001). Under experimental conditions, Daifalla et al. (2011) demonstrated the use of adjuvants, such as the Toll-like receptor 9 (TLR-9) agonist (CpG ODN) or the TLR-4 agonist (GLA-SE), to augment the immunogenicity of the recombinant L. donovani 2-Cys Prx (Prx4) in BALB/c mice. In addition, these immunizations led to increased immune responses to Th1 as high levels of IgG2a antibody titers were induced as well as of IgG1 antibody titers (Daifalla et al. 2011). Thus, rHlPrx2 proteins with CpG ODN and/or GLA-SE could lead to an increased Th1 immune response and might be still considered as vaccine candidates against ticks because of their high immunogenicity.
In summary, we demonstrated that rHlPrx2 could induce high antibody titers of IgG1 related to a Th2 immune response. We also observed that rHlPrx2-immunized mouse sera could recognize native HlPrx2 protein in crude tick midgut proteins by Western blotting. Moreover, the effects of rHlPrx2 immunization in mice were studied using nymphal ticks, but the challenged ticks were not affected by rHlPrx2 immunization. Although the effects of rHlPrx2 immunization did not affect ticks in the present study, rHlPrx2 might still be considered a vaccine candidate against ticks because of its high immunogenicity and the possibility that the combination of Th1 immune response inducible adjuvants might improve our strategy of vaccine development against ticks.
References
Abuodeh RO, Shubitz LF, Siegel E, Snyder S, Peng T, Orsborn KI, Brummer E, Stevens DA, Galgiani JN (1999) Resistance to Coccidioides immitis in mice after immunization with recombinant protein or a DNA vaccine of a proline-rich antigen. Infect Immun 67:2935–2940
Anisuzzamman IM, Alim M, Miyoshi T, Hatta T, Yamaji K, Matsumoto Y, Fujisaki K, Tsuji N (2012) Longistatin is an unconventional serine protease and induces protective immunity against tick infestation. Mol Biochem Parasitol 182:45–53. https://doi.org/10.1016/j.molbiopara.2011.12.002
Brito LA, Singh M (2011) Acceptable levels of endotoxin in vaccine formulations during preclinical research. J Pharm Sci 100:34–37. https://doi.org/10.1002/jps.22267
Cárdenas-Freytag L, Cheng E, Mayeux P, Domer JE, Clements JD (1999) Effectiveness of a vaccine composed of heat-killed Candida albicans and a novel mucosal adjuvant, LT(R192G), against systemic candidiasis. Infect Immun 67:826–833
Citelli M, Lara FA, da Silva VIJ, Oliveira PL (2007) Oxidative stress impairs heme detoxification in the midgut of the cattle tick, Rhipicephalus (Boophilus) microplus. Mol Biochem Parasitol 151:81–88. https://doi.org/10.1016/j.molbiopara.2006.10.008
Daifalla NS, Bayih AG, Gedamu L (2011) Immunogenicity of Leishmania donovani iron superoxide dismutase B1 and peroxidoxin 4 in BALB/c mice: the contribution of Toll-like receptor agonists as adjuvant. Exp Parasitol 129:292–298. https://doi.org/10.1016/j.exppara.2011.07.001
Dalton JP, Robinson MW, Mulcahy G, O’Neill SM, Donnelly S (2013) Immunomodulatory molecules of Fasciola hepatica: candidates for both vaccine and immunotherapeutic development. Vet Parasitol 195:272–285. https://doi.org/10.1016/j.vetpar.2013.04.008
Donnelly S, Stack CM, O’Neill SM, Sayed AA, Williams DL, Dalton JP (2008) Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB J 22:4022–4032. https://doi.org/10.1096/fj.08-106278
Fujisaki K (1978) Development of acquired resistance precipitating antibody in rabbits experimentally infested with females of Haemaphysalis longicornis (Ixodoidea: Ixodidae). Natl Inst Anim Health Q Tokyo 18:27–38
Galay RL, Umemiya-Shirafuji R, Mochizuki M, Fujisaki K, Tanaka T (2015) Iron metabolism in hard ticks (Acari: Ixodidae): the antidote to their toxic diet. Parasitol Int 64:182–189. https://doi.org/10.1016/j.parint.2014.12.005
Grandjean O (1983) Blood digestion in Ornithodorus moubata Murray sensu stricto Walton females (Ixodoidea: Argasidae) II. Modifications of midgut cells related to the digestive cycle and to the triggering action of mating. Ann Parasitol Hum Comp 58:493–514
Gretes MC, Poole LB, Karplus PA (2012) Peroxiredoxins in parasites. Antioxid Redox Signal 17:608–633. https://doi.org/10.1089/ars.2011.4404
Hall A, Nelson K, Poole LB, Karplus PA (2011) Structure-based insights into the catalytic power and conformational dexterity of peroxiredoxins. Antioxid Redox Signal 15:795–815. https://doi.org/10.1089/ars.2010.3624
Kawazu S, Komaki-Yasuda K, Oku H, Kano S (2008) Peroxiredoxins in malaria parasites: parasitologic aspects. Parasitol Int 57:1–7. https://doi.org/10.1016/j.parint.2007.08.001
Kumar D, Budachetri K, Meyers VC, Karim S (2016) Assessment of tick antioxidant responses to exogenous oxidative stressors and insight into the role of catalase in the reproductive fitness of the Gulf Coast tick, Amblyomma maculatum. Insect Mol Biol 25:283–294. https://doi.org/10.1111/imb.12218
Kusakisako K, Masatani T, Miyata T, Galay RL, Maeda H, Talactac MR, Tsuji N, Mochizuki M, Fujisaki K, Tanaka T (2016a) Functional analysis of recombinant 2-Cys peroxiredoxin from the hard tick Haemaphysalis longicornis. Insect Mol Biol 25:16–23. https://doi.org/10.1111/imb.12193
Kusakisako K, Galay RL, Umemiya-Shirafuji R, Hernandez EP, Maeda H, Talactac MR, Tsuji N, Mochizuki M, Fujisaki K, Tanaka T (2016b) 2-Cys peroxiredoxin is required in successful blood-feeding, reproduction, and antioxidant response in the hard tick Haemaphysalis longicornis. Parasit Vectors 9:457. https://doi.org/10.1186/s13071-016-1748-2
Li X, Sambhara S, Li CX, Ewasyshyn M, Parrington M, Caterini J, James O, Cates G, Du RP, Klein M (1998) Protection against respiratory syncytial virus infection by DNA immunization. J Exp Med 188:681–688
Mendes RE, Perez-Ecija RA, Zafra R, Buffoni L, Martinez-Moreno A, Dalton JP, Mulcahy G, Perez J (2010) Evaluation of hepatic changes and local and systemic immune responses in goats immunized with recombinant Peroxiredoxin (Prx) and challenged with Fasciola hepatica. Vaccine 28:2832–2840. https://doi.org/10.1016/j.vaccine.2010.01.055
Miller MA, Skeen MJ, Ziegler HK (1995) Nonviable bacterial antigens administered with IL-12 generate antigen-specific T cell responses and protective immunity against Listeria monocytogenes. J Immunol 155:4817–4828
Mountford AP, Anderson S, Wilson RA (1996) Induction of Th1 cell-mediated protective immunity to Schistosoma mansoni by co-administration of larval antigens and IL-12 as an adjuvant. J Immunol 156:4739–4745
Peterson TM, Luckhart S (2006) A mosquito 2-Cys peroxiredoxin protects against nitrosative and oxidative stresses associated with malaria parasite infection. Free Radic Biol Med 40:1067–1082. https://doi.org/10.1016/j.freeradbiomed.2005.10.059
Raina OK, Nagar G, Varghese A, Prajitha G, Alex A, Maharana BR, Joshi P (2011) Lack of protective efficacy in buffaloes vaccinated with Fasciola gigantica leucine aminopeptidase and peroxiredoxin recombinant proteins. Acta Trop 118:217–222. https://doi.org/10.1016/j.actatropica.2011.02.008
Robinson MW, Hutchinson AT, Dalton JP, Donnelly S (2010) Peroxiredoxin: a central player in immune modulation. Parasite Immunol 32:305–313. https://doi.org/10.1111/j.1365-3024.2010.01201.x
Rudenko N, Golovchenko M, Edwards MJ, Grubhoffer L (2005) Differential expression of Ixodes ricinus tick genes induced by blood feeding or Borrelia burgdorferi infection. J Med Entomol 42:36–41
Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh T, Honma K, Matsuyama T, Yui K, Tsujimura T, Standley DM, Nakanishi K, Nakai K, Akira S (2010) The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol 11:936–944. https://doi.org/10.1038/ni.1920
Sekiya M, Mulcahy G, Irwin JA, Stack CM, Donnelly SM, Xu W, Collins P, Dalton JP (2006) Biochemical characterisation of the recombinant peroxiredoxin (FhePrx) of the liver fluke, Fasciola hepatica. FEBS Lett 580:5016–5022. https://doi.org/10.1016/j.febslet.2006.08.019
Spellberg B, Edwards JE Jr (2001) Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis 32:76–102
Tanaka T, Kusakisako K, Miyata K (2016) The development of anti-tick vaccine as a target against regulating molecule for oxidant stress. In: Final reports for research grants for meat and meat products, vol 34. The Ito Foundation, Tokyo, pp 253–257 (in Japanese)
Tsuji N, Kamio T, Isobe T, Fujisaki K (2001) Molecular characterization of a peroxiredoxin from the hard tick Haemaphysalis longicornis. Insect Mol Biol 10:121–129
Umemiya-Shirafuji R, Tanaka T, Boldbaatar D, Tanaka T, Fujisaki K (2012) Akt is an essential player in regulating cell/organ growth at the adult stage in the hard tick Haemaphysalis longicornis. Insect Biochem Mol Biol 42:164–173
Wood ZA, Poole LB, Hantgan RR, Karplus PA (2002) Dimers to doughnuts: redox-sensitive oligomerization of 2-cysteine peroxiredoxins. Biochemistry 41:5493–5504
Yanase N, Toyota H, Hata K, Yagyu S, Seki T, Harada M, Kato Y, Mizuguchi J (2014) OVA-bound nanoparticles induce OVA-specific IgG1, IgG2a, and IgG2b responses with low IgE synthesis. Vaccine 32:5918–5924. https://doi.org/10.1016/j.vaccine.2014.08.059
Acknowledgements
This study was funded by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 15H05264, 16H05028, and 16J08221, and, in part, by a Grant from The Ito Foundation, Japan. K. Kusakisako is supported by a Grant-in-Aid for JSPS fellows.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kusakisako, K., Miyata, T., Tsujio, M. et al. Evaluation of vaccine potential of 2-Cys peroxiredoxin from the hard tick Haemaphysalis longicornis. Exp Appl Acarol 74, 73–84 (2018). https://doi.org/10.1007/s10493-018-0209-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-018-0209-3