Abstract
Introduction
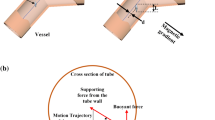
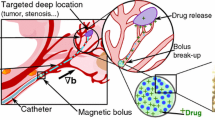
Magnetic resonance navigation (MRN) uses MRI gradients to steer magnetic drug-eluting beads (MDEBs) across vascular bifurcations. We aim to experimentally verify our theoretical forces balance model (gravitational, thrust, friction, buoyant and gradient steering forces) to improve the MRN targeted success rate.
Method
A single-bifurcation phantom (3 mm inner diameter) made of poly-vinyl alcohol was connected to a cardiac pump at 0.8 mL/s, 60 beats/minutes with a glycerol solution to reproduce the viscosity of blood. MDEB aggregates (25 ± 6 particles, 200 \(\mu {\text{m}}\)) were released into the main branch through a 5F catheter. The phantom was tilted horizontally from − 10° to +25° to evaluate the MRN performance.
Results
The gravitational force was equivalent to 71.85 mT/m in a 3T MRI. The gradient duration and amplitude had a power relationship (amplitude=78.717 \({(duration)}^{-0.525}\)). It was possible, in 15° elevated vascular branches, to steer 87% of injected aggregates if two MRI gradients are simultaneously activated (\({G}_{x}\) = +26.5 mT/m, \({G}_{y}\)= +18 mT/m for 57% duty cycle), the flow velocity was minimized to 8 cm/s and a residual pulsatile flow to minimize the force of friction.
Conclusion
Our experimental model can determine the maximum elevation angle MRN can perform in a single-bifurcation phantom simulating in vivo conditions.








Similar content being viewed by others
Data and Materials Availability
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
Abbreviations
- \(\overrightarrow{B0}\) :
-
Main MRI magnetic field strength
- EPI:
-
Echo planar readout
- MDEBs:
-
Magnetic drug-eluting beads
- MRN:
-
Magnetic resonance navigation
- MRI:
-
Magnetic resonance imaging
- PLGA:
-
Poly-lactic-co-glycolic acid
- PVA:
-
Poly-vinyl alcohol
- T2:
-
Relaxation time of the transverse magnetization
- TE:
-
Echo time
- TR:
-
Repetition time
References
Abolfathi, K., M. R. H. Yazdi, and A. K. Hoshiar. Studies of different swarm modes for the MNPs under the rotating magnetic field. IEEE Trans. Nanotechnol. 19:849–855, 2020.
Azizi, A., C. C. Tremblay, K. Gagné, and S. Martel. Using the fringe field of a clinical MRI scanner enables robotic navigation of tethered instruments in deeper vascular regions. Sci. Robot. 4:7342, 2019.
Bigot, A., G. Soulez, and S. Martel. A prototype of injector to control and to detect the release of magnetic beads within the constraints of multibifurcation magnetic resonance navigation procedures. Magn. Reson. Med. 77:444–452, 2017.
Bigot, A., C. Tremblay, G. Soulez, and S. Martel. Magnetic resonance navigation of a bead inside a three-bifurcation PMMA phantom using an imaging gradient coil insert. IEEE Trans. Robot. 30:719–727, 2014.
Cheng, N.-S. Formula for the viscosity of a glycerol−water mixture. Ind. Eng. Chem. Res. 47:3285–3288, 2008.
Erin, O., M. Boyvat, J. Lazovic, M. E. Tiryaki, and M. Sitti. Wireless MRI-powered reversible orientation-locking capsule robot. Adv. Sci. 2021. https://doi.org/10.1002/advs.202100463.
Felfoul, O., A. T. Becker, G. Fagogenis, and P. E. Dupont. Simultaneous steering and imaging of magnetic particles using MRI toward delivery of therapeutics. Sci. Rep. 6:33567, 2016.
Folio, D., and A. Ferreira. Two-dimensional robust magnetic resonance navigation of a ferromagnetic microrobot using pareto optimality. IEEE Trans. Robot. 33:583–593, 2017.
Gaba, R. C., R. P. Lokken, R. M. Hickey, A. J. Lipnik, R. J. Lewandowski, R. Salem, D. B. Brown, T. G. Walker, J. E. Silberzweig, M. O. Baerlocher, A. M. Echenique, M. Midia, J. W. Mitchell, S. A. Padia, S. Ganguli, T. J. Ward, J. L. Weinstein, B. Nikolic, and S. R. Dariushnia. Society of interventional radiology standards of practice committee quality improvement guidelines for transarterial chemoembolization and embolization of hepatic malignancy. J. Vasc. Interv. Radiol. 28:1210–1223, 2017.
Hoshiar, A. K., T.-A. Le, P. Valdastri, and J. Yoon. Swarm of magnetic nanoparticles steering in multi-bifurcation vessels under fluid flow. J. Micro-Bio Robot. 16:137–145, 2020.
Jackson, J. D., and J. Wiley. Classical Electrodynamics Third Editionat. https://cds.cern.ch/record/490457/files/9780471309321_TOC.pdf
Jiang, J., L. Yang, and L. Zhang. Closed-loop control of a Helmholtz coil system for accurate actuation of magnetic microrobot swarms. IEEE Robot. Autom. Lett. 6:827–834, 2021.
Kosukegawa, H., K. Mamada, K. Kuroki, L. Liu, K. Inoue, T. Hayase, and M. Ohta. Measurements of dynamic viscoelasticity of poly (vinyl alcohol) hydrogel for the development of blood vessel biomodeling*. J. Fluid Sci. Technol. 3:533, 2008.
Latulippe, M., and S. Martel. Dipole field navigation: theory and proof of concept. IEEE Trans. Robot. 31:1353–1363, 2015.
Li, N., Y. Jiang, R. Plantefève, F. Michaud, Z. Nosrati, C. Tremblay, K. Saatchi, U. O. Häfeli, S. Kadoury, G. Moran, F. Joly, S. Martel, and G. Soulez. Magnetic resonance navigation for targeted embolization in a two-level bifurcation phantom. Ann. Biomed. Eng. 47:2402–2415, 2019.
Li, N., F. Michaud, Z. Nosrati, D. Loghin, C. Tremblay, R. Plantefeve, K. Saatchi, U. Hafeli, S. M. Martel, and G. Soulez. MRI-compatible injection system for magnetic microparticle embolization. IEEE Trans. Biomed. Eng. 2018. https://doi.org/10.1109/TBME.2018.2889000.
Li, N., C. Tremblay, and S. Martel. Combining oscillating flow & clinical MRI gradients for targeted therapy. IEEE. 2017. https://doi.org/10.1109/MARSS.2017.8001937.
Martel, S. Combining pulsed and DC gradients in a clinical MRI-based microrobotic platform to guide therapeutic magnetic agents in the vascular network. Int. J. Adv. Robot. Syst. 10:30, 2013.
Martel, S., C. Tremblay, et al., 2006. Controlled manipulation and actuation of micro-objects with magnetotactic bacteria. aip.scitation.org, 2006. https://aip.scitation.org/doi/abs/https://doi.org/10.1063/1.2402221.
Mellal, L., K. Belharet, D. Folio, and A. Ferreira. Optimal structure of particles-based superparamagnetic microrobots: application to MRI guided targeted drug therapy. J. Nanopart. Res. 2015. https://doi.org/10.1007/s11051-014-2733-3.
Michaud, F., N. Li, R. Plantefève, Z. Nosrati, C. Tremblay, K. Saatchi, G. Moran, A. Bigot, U. O. Häfeli, S. Kadoury, A. Tang, P. Perreault, S. Martel, and G. Soulez. Selective embolization with magnetized microbeads using magnetic resonance navigation in a controlled-flow liver model. Med. Phys. 46:789–799, 2019.
Nosrati, Z., N. Li, F. Michaud, S. Ranamukhaarachchi, S. Karagiozov, G. Soulez, S. Martel, K. Saatchi, and U. O. Häfeli. Development of a coflowing device for the size-controlled preparation of magnetic-polymeric microspheres as embolization agents in magnetic resonance navigation technology. ACS Biomater. Sci. Eng. 4:1092–1102, 2018.
Ouedrago I. Etude de l’anatomie artérielle hépatique chez des patients a eints de CHC, pour un traitement par chimioembolisation par navigation par résonance magnétique, 2019.
Peng Cao, Y., G. Duhamel, B. Ramond. Olympe, and F. Langevin. A new production method of elastic silicone carotid phantom based on MRI acquisition using rapid prototy** technique. Annu Int Conf IEEE Eng Med Biol Soc. 2013. https://doi.org/10.1109/EMBC.2013.6610753.
Polanczyk, A., M. Podgorski, M. Polanczyk, A. Piechota-Polanczyk, C. Neumayer, and L. Stefanczyk. A Novel Patient-Specific Human Cardiovascular System Phantom (HCSP) for reconstructions of pulsatile blood hemodynamic inside abdominal aortic aneurysm. IEEE Access. 6:61896–61903, 2018.
Polanczyk, A., M. Podgorski, M. Polanczyk, A. Piechota-Polanczyk, L. Stefanczyk, and M. Strzelecki. A novel vision-based system for quantitative analysis of abdominal aortic aneurysm deformation. Biomed. Eng. Online. 18:56, 2019.
Pouponneau, P., G. Bringout, and S. Martel. Therapeutic magnetic microcarriers guided by magnetic resonance navigation for enhanced liver chemoembilization: a design review. Ann. Biomed. Eng. 42:929–939, 2014.
Pouponneau, P., J.-C. Leroux, G. Soulez, L. Gaboury, and S. Martel. Co-encapsulation of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation. Biomaterials. 32:3481–3486, 2011.
Suwa, M., and H. Watarai. Magnetoanalysis of micro/nanoparticles: a review. Anal. Chim. Acta. 690:137–147, 2011.
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC), Operating Grant - CHRP (CIHR Partnered) (CHRP 478474-15), Canadian Institutes of Health Research (CIHR), Operating Grant - CHRP (NSERC Partnered) (CPG-140179), Fonds de recherche du Québec en Santé (FRQS) and Fondation de l'association des radiologistes du Québec (FARQ) Clinical Research Scholarship (34939), Transmedtech and Siemens Healthineers. We also acknowledge support by the Lundbeck Foundation of Denmark (UBC-SUND Lundbeck Foundation Professorship to UOH, No. 2014-4176).
Author information
Authors and Affiliations
Contributions
GS and SL supervised the studies. CT and NL implemented the methods and analysis. All authors edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
C.T is currently supported by Transmedtech and Siemens Healthineers, Canada. G.M and M.J.C work at Siemens Healthineers. The other authors declare that they have no competing financial interests.
Additional information
Associate Editor Stefan M Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file5 (MP4 6898 KB)
Rights and permissions
About this article
Cite this article
Tous, C., Li, N., Dimov, I.P. et al. Navigation of Microrobots by MRI: Impact of Gravitational, Friction and Thrust Forces on Steering Success. Ann Biomed Eng 49, 3724–3736 (2021). https://doi.org/10.1007/s10439-021-02865-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-021-02865-1