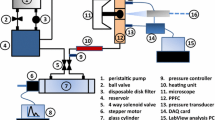
Abstract
Arterial flow characteristics determine vessel health by modulating vascular endothelial cells. One system used to study these interactions is the parallel plate flow chamber. The present in vitro study quantified the uniformity of fluid flow across a parallel plate flow chamber and characterized plate-location dependent endothelial cell gene expression. More specifically, shear stress varied by as much as 11% across the chamber area, which caused non-uniform ecNOS (p < 0.05) and COX-2 (p < 0.05) mRNA expression across the plate area. Results herein suggest that chamber variations may result during construction or assembly, which ultimately affect flow-sensitive cell responses (including mRNA expression). Therefore, these limitations should be considered when reporting endothelial cell responses to fluid flow using parallel plate flow chambers.
Similar content being viewed by others
References
Barbee, K. A., P. F. Davies, and R. Lal. Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. Circ. Res. 74:163–171, 1994.
Barbee, K. A. Role of subcellular shear-stress distributions in endothelial cell mechanotransduction. Ann. Biomed. Eng. 30:472–482, 2002.
Chung, B. J., A. M. Robertson, and D. G. Peters. The numerical design of a parallel plate flow chamber for investigation of endothelial cell response to shear stress. Comp. Structs. 81:535–546, 2003.
Davies, P. F., T. Mundel, and K. A. Barbee. A mechanism for heterogeneous endothelial responses to flow in vivo and in vitro. J. Biomechan. 28(12):1553–1560, 1995.
Devasenathipathy, S., J. G. Santiago, S. T. Wereley, and C. D. Meinhart. Particle imaging techniques for microfabricated fluidic systems. Exp. Fluids. 34(4):504–514, 2003.
Dieterich, P., M. Odenthal-Schnittler, C. Mrowietz, M. Kramer, L. Sasse, H. Oberleithner, and H. J. Schnittler. Quantitative morphodynamic of endothelial cells within confluent cultures in response to fluid shear stress. Biophys. J. 79:1285–1297, 2000.
Dring, R. P. Sizing criteria for laser anemometry particles. J. Fluids Eng. 104(1):15–17, 1982.
Frangos, J. A., L. V. McIntire, and S. G. Eskin. Shear stress stimulation of mammalian cell metabolism. Biotechnol. Bioeng. 32:1053–1060, 1988.
Galbraith, C. G., R. Skalak, and S. Chien. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil. Cytoskeleton 40:317–330, 1998.
Gomez, R., R. Bashir, A. Sarakaya, M. R. Ladisch, J. Sturgis, J. P. Robinson, T. Geng, A. K. Bhunia, H. L. Apple, and S. T. Wereley. Microfluidic biochip for impedance spectroscopy of biological species. Biomed. Microdev. 3:258–268, 2001.
Hirafuji, M., M. Tsunoda, T. Machida, N. Hamaue, T. Endo, A. Miyamoto, and M. Minami. Reduced expressions of inducible nitric oxide synthase and cyclooxygenase-2 in vascular smooth muscle cells of stroke-prone spontaneously hypertensive rats. Life Sci. 70:917–926, 2002.
Ignarro, L. J., G. M. Buga, L. H. Wei, P. M. Bauer, G. Wu, and P. del Soldato. Role of arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc. Natl. Acad. Sci. U.S.A. 98:4202–4208, 2001.
Imberti, B., M. Morigi, C. Zoja, S. Angioletti, M. Abbate, A. Remuzzi, and G. Remuzzi. Shear stress-induced cytoskeleton rearrangement mediates NF-κB-dependent endothelial expression of ICAM-1. Microvasc. Res. 60:182–188, 2000.
Lee, S. Y., S. T. Wereley, L. C. Gui, W. L. Qu, and I. Mudawar. Microchannel flow measurement using micro particle image velocimetry. Proc. ASME/IMECE. Paper #2002–33682, New Orleans, LA. 2002.
Malek, A. M., S. L. Apler, and S. I. Izumo. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282:2035–2042, 1999.
Meinhart, C. D., S. T. Wereley, and J. G. Santiago. PIV measurements of a microchannel flow. Exp. Fluids. 27:414–419, 1999.
Meinhart, C. D., S. T. Wereley, and J. G. Santiago. Micron-Resolution Velocimetry Techniques. Laser Techniques Applied to Fluid Mechanics, edited by R. J. Adrian et al. Berlin: Springer-Verlag, 2000.
Nauman, E. A., K. J. Risic, T. M. Keaveny, and R. L. Satcher. Quantitative assessment of steady and pulsatile flow fields in a parallel plate flow chamber. Ann. Biomed. Eng. 27:194–199, 1999.
Nerem, R. M., P. R. Girard, G. Helmlinger, O. Thoumine, T. F. Wiesner, and T. Ziegler. Cell Mechanics and Cellular Engineering. edited by V. C. Mow, F. Guilak, R. Tran-Son-Tay, and R. M. Hochmuth. New York: Springer-Verlag, 1994, Chap. 5.
Raffel, M., C. Willert, and J. Kompenhans. Particle Image Velocimetry: A Practical Guide. Springer Press, Germany, 1998.
Resnick, N., H. Yahav, S. Schubert, E. Wolfovitz, and A. Shay. Signalling pathways in vascular endothelium activated by shear stress: Relevance to atherosclerosis. Curr. Opin. Lipidol. 11:167–177, 2000.
Ross, R. Endothelial Dysfunctions. New York: Plenum Press, 1992, Chapter 18.
Samimy, M. and S. K. Lele. Motion of particles with inertia in a compressible free shear flow. Phys. Fluids A 3:1915–1923, 1991.
Santiago, J. G., S. T. Wereley, C. D. Meinhart, D. J. Beebe, and R. J. Adrian. A particle image velocimetry system for microfluidics. Exp. Fluids. 25:316–319, 1998.
Tardy, Y., N. Resnick, T. Nagel, and M. A. Gimbrone Jr. Shear stress gradients remodel endothelial monolayers in vitro via a cell proliferation-migration-loss cycle. Arterioscler. Thromb. Vasc. Biol. 17:3102–3106, 1997.
Timoshenko, S. Theory of Plates and Shells. NewYork: McGraw-Hill, 1940.
Topper, J. N. and M. A. Gimbrone Jr. Blood flow and vascular gene expression: Fluid shear stress as a modulator of endothelial cell phenotype. Mol. Med. Today 5:40–46, 1999.
Topper, J. N., J. Cai, D. Falb, and M. A. Gimbrone. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: Cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar flow. Proc. Natl. Acad. Sci. U.S.A. 93:10417–10422, 1996.
Traub, O. and B. C. Berk. Laminar shear stress mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler. Thromb. Vasc. Biol. 18:677–685, 1998.
Ukropec, J. A., M. K. Hollinger, and M. J. Woolkalis. Regulation of VE-cadherin linkage to the cytoskeleton in endothelial cells exposed to fluid shear stress. Exp. Cell Res. 273:240–247, 2002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCann, J.A., Peterson, S.D., Plesniak, M.W. et al. Non-Uniform Flow Behavior in a Parallel Plate Flow Chamber Alters Endothelial Cell Responses. Ann Biomed Eng 33, 328–336 (2005). https://doi.org/10.1007/s10439-005-1735-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10439-005-1735-9