Abstract
Purpose
Subthreshold micropulse laser (SMPL) is more clinically efficient for the treatment of diabetic macular edema (DME) than the conventional continuous-wave (CW) laser. We aimed to characterize transcriptome changes after the application of these lasers and to compare the transcripts.
Methods
Human pluripotent stem cell-derived retinal pigment epithelial cells were exposed to laser irradiation. Differentially expressed genes (DEGs), distribution of heat shock protein (Hsp) family, gene expression profile, and gene ontology (GO) enrichment analysis based on RNA sequencing data were investigated at 3 h and 24 h after irradiation.
Results
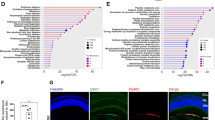
CW laser induced more DEGs than SMPL (1771 vs. 520 genes). The expression of the Hsp family was confirmed in both groups: however, the induction patterns was different for different genes. GO enrichment analysis revealed that CW laser upregulated the expression of DEGs involved in vasculature development (GO: 0001944), related to apoptosis and repair after cell injury whereas SMPL upregulated the expression of DEGs involved in photoreceptor cell maintenance (GO: 0045494), photoreceptor cell development (GO: 0042461), and sensory perception of light stimuli (GO: 0050953).
Conclusions
The results provide insights into the genetic responses and may contribute to the understanding of the molecular mechanisms of laser-induced thermal effects.


Similar content being viewed by others
References
Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–806.
Schatz H, Madeira D, McDonald HR, Johnson RN. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol. 1991;109:1549–51.
Guyer DR, D’Amico DJ, Smith CW. Subretinal fibrosis after laser photocoagulation for diabetic macular edema. Am J Ophthalmol. 1992;113:652–6.
Writing Committee for the Diabetic Retinopathy Clinical Research N, Fong DS, Strauber SF, Aiello LP, Beck RW, Callanan DG, et al. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125:469–80.
Hudson C, Flanagan JG, Turner GS, Chen HC, Young LB, McLeod D. Influence of laser photocoagulation for clinically significant diabetic macular oedema (DMO) on short-wavelength and conventional automated perimetry. Diabetologia. 1998;41:1283–92.
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25.
Ishibashi T, Li X, Koh A, Lai TY, Lee FL, Lee WK, et al. The REVEAL Study: Ranibizumab Monotherapy or Combined with Laser versus Laser Monotherapy in Asian Patients with Diabetic Macular Edema. Ophthalmology. 2015;122:1402–15.
Friberg TR, Karatza EC. The treatment of macular disease using a micropulsed and continuous wave 810-nm diode laser. Ophthalmology. 1997;104:2030–8.
Terasaki H, Ogura Y, Kitano S, Sakamoto T, Murata T, Hirakata A, et al. Management of diabetic macular edema in Japan: a review and expert opinion. Jpn J Ophthalmol. 2018;62:1–23.
Lai FHP, Chan RPS, Lai ACH, Tsang S, Woo TTY, Lam RF, et al. Comparison of two-year treatment outcomes between subthreshold micropulse (577 nm) laser and aflibercept for diabetic macular edema. Jpn J Ophthalmol. 2021;65:680–8.
Takatsuna Y, Yamamoto S, Nakamura Y, Tatsumi T, Arai M, Mitamura Y. Long-term therapeutic efficacy of the subthreshold micropulse diode laser photocoagulation for diabetic macular edema. Jpn J Ophthalmol. 2011;55:365–9.
Inagaki K, Ohkoshi K, Ohde S, Deshpande GA, Ebihara N, Murakami A. Comparative efficacy of pure yellow (577-nm) and 810-nm subthreshold micropulse laser photocoagulation combined with yellow (561–577-nm) direct photocoagulation for diabetic macular edema. Jpn J Ophthalmol. 2015;59:21–8.
Qiao G, Guo HK, Dai Y, Wang XL, Meng QL, Li H, et al. Sub-threshold micro-pulse diode laser treatment in diabetic macular edema: a Meta-analysis of randomized controlled trials. Int J Ophthalmol. 2016;9:1020–7.
Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89:74–80.
Lavinsky D, Cardillo JA, Melo LA Jr, Dare A, Farah ME, Belfort R Jr. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci. 2011;52:4314–23.
Chen G, Tzekov R, Li W, Jiang F, Mao S, Tong Y. Subthreshold micropulse diode laser versus conventional laser photocoagulation for diabetic macular edema: a meta-analysis of randomized controlled trials. Retina. 2016;36:2059–65.
Figueira J, Khan J, Nunes S, Sivaprasad S, Rosa A, de Abreu JF, et al. Prospective randomised controlled trial comparing sub-threshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. Br J Ophthalmol. 2009;93:1341–4.
Ohkoshi K, Yamaguchi T. Subthreshold micropulse diode laser photocoagulation for diabetic macular edema in Japanese patients. Am J Ophthalmol. 2010;149:133–9.
Whitley D, Goldberg SP, Jordan WD. Heat shock proteins: a review of the molecular chaperones. J Vasc Surg. 1999;29:748–51.
Inagaki K, Shuo T, Katakura K, Ebihara N, Murakami A, Ohkoshi K. Sublethal photothermal stimulation with a micropulse laser induces heat shock protein expression in ARPE-19 cells. J Ophthalmol. 2015;2015: 729792.
Inagaki K, Hamada M, Ohkoshi K. Minimally invasive laser treatment combined with intravitreal injection of anti-vascular endothelial growth factor for diabetic macular oedema. Sci Rep. 2019;9:7585.
Tripathi S, Pohl MO, Zhou Y, Rodriguez-Frandsen A, Wang G, Stein DA, et al. Meta- and orthogonal integration of influenza “OMICs” data defines a role for UBR4 in virus budding. Cell Host Microbe. 2015;18:723–35.
Tababat-Khani P, de la Torre C, Canals F, Bennet H, Simo R, Hernandez C, et al. Photocoagulation of human retinal pigment epithelium in vitro: unravelling the effects on ARPE-19 by transcriptomics and proteomics. Acta Ophthalmol. 2015;93:348–54.
Lavinsky D, Wang J, Huie P, Dalal R, Lee SJ, Lee DY, et al. Nondamaging retinal laser therapy: rationale and applications to the macula. Invest Ophthalmol Vis Sci. 2016;57:2488–500.
Wang J, Quan Y, Dalal R, Palanker D. Comparison of continuous-wave and micropulse modulation in retinal laser therapy. Invest Ophthalmol Vis Sci. 2017;58:4722–32.
Kern K, Mertineit CL, Brinkmann R, Miura Y. Expression of heat shock protein 70 and cell death kinetics after different thermal impacts on cultured retinal pigment epithelial cells. Exp Eye Res. 2018;170:117–26.
Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. 2008;14:342–6.
van der Noll R, Leijen S, Neuteboom GH, Beijnen JH, Schellens JH. Effect of inhibition of the FGFR-MAPK signaling pathway on the development of ocular toxicities. Cancer Treat Rev. 2013;39:664–72.
Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737.
Yao B, Wang S, **ao P, Wang Q, Hea Y, Zhang Y. MAPK signaling pathways in eye wounds: multifunction and cooperation. Exp Cell Res. 2017;359:10–6.
Tababat-Khani P, Berglund LM, Agardh CD, Gomez MF, Agardh E. Photocoagulation of human retinal pigment epithelial cells in vitro: evaluation of necrosis, apoptosis, cell migration, cell proliferation and expression of tissue repairing and cytoprotective genes. PLoS ONE. 2013;8: e70465.
Wu H, Wang G, Li S, Zhang M, Li H, Wang K. TNF-alpha- mediated-p38-dependent signaling pathway contributes to myocyte apoptosis in rats subjected to surgical trauma. Cell Physiol Biochem. 2015;35:1454–66.
ElKeeb AM, Collier ME, Maraveyas A, Ettelaie C. Accumulation of tissue factor in endothelial cells induces cell apoptosis, mediated through p38 and p53 activation. Thromb Haemost. 2015;114:364–78.
Maeshima K, Utsugi-Sutoh N, Otani T, Kishi S. Progressive enlargement of scattered photocoagulation scars in diabetic retinopathy. Retina. 2004;24:507–11.
Pan L, Zhao Y, Yuan Z, Qin G. Research advances on structure and biological functions of integrins. Springerplus. 2016;5:1094.
Blandin AF, Noulet F, Renner G, Mercier MC, Choulier L, Vauchelles R, et al. Glioma cell dispersion is driven by alpha5 integrin-mediated cell-matrix and cell-cell interactions. Cancer Lett. 2016;376:328–38.
De Cilla S, Vezzola D, Farruggio S, Vujosevic S, Clemente N, Raina G, et al. The subthreshold micropulse laser treatment of the retina restores the oxidant/antioxidant balance and counteracts programmed forms of cell death in the mice eyes. Acta Ophthalmol. 2019;97:e559–67.
Acknowledgements
This work was supported by JSPS KAKENHI (Grants-in-Aid for Scientific Research) Grant Number 19K18872. Professional medical English editing was done by Editage.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
T. Shiraya, None; F. Araki, None; S. Nakagawa, None; T. Ueta, None; K. Totsuka, None; H. Abe, None; Y. Naito, None; T. Toyama, None; K. Sugimoto, None; S. Kato, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding author: Satoshi Kato
Supplementary Information
Below is the link to the electronic supplementary material.
10384_2022_925_MOESM1_ESM.tif
Supplementary file1 Supplementary material. Experimental setup for laser irradiation of human pluripotent stem cell-derived RPE cells. a. The figure shows RPE cells immediately after irradiation with CW laser at a power ranging from 100 to 300 mW. Lesions formed by CW laser provided at a power ranging from 140 to 160 mW are ophthalmoscopically visible (arrowheads). The bottom row shows intentional burns, which are used to identify the irradiation site at each power. Cell viability was assessed using a LIVE/DEADTM Cell Imaging kit. Live cells were able to hydrolyze calcein-AM and were therefore stained green, while dead cells were permeable to ethidium homodimer-1 and were therefore stained red. b. Results of cell viability assay after two hours of CW laser irradiation are shown in Figure (a). Interestingly, cell death was also observed at 100-130 mW, where no coagulation spots were observed. c. Cell viability assay after two hours of SMPL irradiation revealed, no cellular death below 300-mW exposure, minimal cellular death at 400 mW and obvious cellular death at 500 mW. The bottom row shows intentional burns, which are used to identify the irradiation site at each power. d. Quantitative analysis showed that the expression of Hsp70 mRNA (n = 3) increased over baseline at 300 and 500 mW. **p < 0.01 compared to the non-irradiated control. The primers for Hsp70 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were as follows: forward (ATGTCGGTGGTGGGCATAGA), reverse (CACAGCGACGTAGCAGCTCT); forward (GAGTCAACGGATTTGGTCGT), reverse (TTGATTTTGGAGGGATCTCG). Abbreviations: CW, continuous-wave; SMPL, subthreshold micropulse laser; Hsp, heat shock protein (TIF 2505 KB)
About this article
Cite this article
Shiraya, T., Araki, F., Nakagawa, S. et al. Differential gene expression analysis using RNA sequencing: retinal pigment epithelial cells after exposure to continuous-wave and subthreshold micropulse laser. Jpn J Ophthalmol 66, 487–497 (2022). https://doi.org/10.1007/s10384-022-00925-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-022-00925-0