Abstract
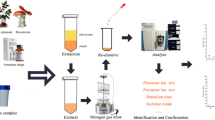
In the present study, liquid chromatography coupled to an Orbitrap mass spectrometer (HPLC–Q-Orbitrap MS) was used as an approach for identification and quantification of 113 drugs simultaneously in biological samples (whole blood/plasma/serum). Samples were prepared using liquid–liquid extraction conducted using a trizma/isopropanol/butyl chloride buffer system. Reversed-phase separation employing a column (50 × 2.1 mm) packed with 2.6-μm C18 particles was then performed under gradient elution with mobile phase composition consisting of acetic acid and aqueous-acetonitrile mixtures with the acetonitrile content ranging from 10 to 100% v/v. Compounds were detected with high-resolution MS operated in full scan mode having a mass accuracy < 5 ppm. In this study, isobaric compounds (same nominal mass) were easily distinguished and identified by their different retention times. Extracted ion chromatograms (XICs) with narrow mass tolerance window (5 ppm) provided analysis with acceptable linearity (r2) ranged from 0.9530 to 1, low limits of detection (LOD) (0.02–39 ng mL−1) and low limit of quantification (LOQ) (0.1–130 ng mL−1). The developed method was applied to successfully analyse drugs in 26 blood samples from positive forensic cases and proved that this technique was able to detect analytes at trace level.
Graphic Abstract





Similar content being viewed by others
References
Paterson S, Cordero R, Burlinson S (2004) Screening and semi-quantitative analysis of post mortem blood for basic drugs using gas chromatography/ion trap mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 813:323–330
Guinan TM, Kirkbride P, Della Vedova CB, Kershaw SG, Kobus H, Voelcker NH (2015) Direct detection of illicit drugs from biological fluids by desorption/ionization mass spectrometry with nanoporous silicon microparticles. Analyst 140(23):7926–7933
Liu P, Hu Y, Chen J, Yang Q (2015) Direct detection of the anti-cancer drug 9-phenylacridine in tissues by graphite rod laser desorption vacuum-ultraviolet post-ionization mass spectrometry. Rapid Commun Mass Spectrom 29(14):1328–1334
Leung GNW, Chung EW, Ho ENM, Kwok WH, Leung DKK, Tang FPW, Wan TSM, Yu NH (2005) High-throughput screening of corticosteroids and basic drugs in horse urine by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 825:47–56
Peters FT (2011) Recent advances of liquid chromatography-(tandem) mass spectrometry in clinical and forensic toxicology. Clin Biochem 44:54–65
Lynch KL, Breaud AR, Vandenberghe H, Wu AHB, Clarke W (2010) Performance evaluation of three liquid chromatography mass spectrometry methods for broad spectrum drug screening. Clin Chim Acta 411:1474–1481
Remane D, Meyer MR, Peters FT, Wissenbach DK, Maurer HH (2010) Fast and simple procedure for liquid–liquid extraction of 136 analytes from different drug classes for development of a liquid chromatographic-tandem mass spectrometric quantification method in human blood plasma. Anal Bioanal Chem 397:2303–2314
Di Rago M, Saar E, Rodda LN, Turfus S, Kotsos A, Gerostamoulos D, Drummer OH (2014) Fast targeted analysis of 132 acidic and neutral drugs and poisons in whole blood using LC–MS/MS. Forensic Sci Int 243:35–43
Moulard Y, Bailly-Chouriberry L, Boyer S, Garcia P, Popot MA, Bonnaire Y (2011) Use of benchtop exactive high resolution and high mass accuracy orbitrap mass spectrometer for screening in horse do** control. Anal Chim Acta 700:126–136
Ojanperä I, Kolmonen M, Pelander A (2012) Current use of high-resolution mass spectrometry in drug screening relevant to clinical and forensic toxicology and do** control. Anal Bioanal Chem 403:1203–1220
Boix C, Ibáñez M, Sancho JV, León N, Yusá V, Hernández F (2014) Qualitative screening of 116 veterinary drugs in feed by liquid chromatography-high resolution mass spectrometry: potential application to quantitative analysis. Food Chem 160:313–320
Vogliardi S, Favretto D, Tucci M, Stocchero G, Ferrara SD (2011) Simultaneous LC-HRMS determination of 28 benzodiazepines and metabolites in hair. Anal Bioanal Chem 400:51–67
Broeker S, Herre S, Wust B, Zweigenbaum J, Pragst F (2011) Development and practical application of a library of CID accurate mass spectra of more than 2500 toxic compounds for systematic toxicological analysis by LC-QTOF-MS with data dependent acquistion. Anal Bioanal Chem 400:101–117
Pelander A, Ojanperä I, Laks S, Rasanen I, Vuori E (2003) Toxicological screening with formula-based metabolite identification by liquid chromatography/time-of-flight mass spectrometry. Anal Chem 75:5710–5718
Thomas A, Guddat S, Kohler M, Krug O, Schänzer W, Petrou M, Thevis M (2010) Comprehensive plasma-screening for known and unknown substances in do** controls. Rapid Commun Mass Spectrom 24:1124–1132
Gómez-Pérez ML, Romero-González R, Plaza-Bolaños P, Génin E, Vidal JLM, Frenich AG (2014) Wide-scope analysis of pesticide and veterinary drug residues in meat matrices by high resolution MS: detection and identification using Exactive-Orbitrap. J Mass Spectrom 49:27–36
Strano-Rossi S, Odoardi S, Castrignano E, Serpelloni G, Chiarotti M (2015) Liquid chromatography-high resolution mass spectrometry (LC–HRMS) determination of stimulants, anorectic drugs and phosphodiesterase 5 inhibitors (PDE5I) in food supplements. J Pharm Biomed Anal 106:144–152
Makarov A (2000) Electrostatic axially harmonic orbital trap**: a high-performance technique of mass analysis. Anal Chem 72:1156–1162
Concheiro M, Castaneto M, Kronstrand R, Huestis MA (2015) Simultaneous determination of 40 novel psychoactive stimulants in urine by liquid chromatography-high resolution mass spectrometry and library matching. J Chromatogr A 1397:32–42
Marzinke MA, Breaud A, Parsons TL, Cohen MS, Piwowar-Manning E, Eshleman SH, Clarke W (2014) The development and validation of a method using high-resolution mass spectrometry (HRMS) for the qualitative detection of antiretroviral agents in human blood. Clin Chim Acta 433:157–168
Thomas A, Geyer H, Schänzer W, Crone C, Kellmann M, Moehring T, Thevis M (2012) Sensitive determination of prohibited drugs in dried blood spots (DBS) for do** controls by means of a benchtop quadrupole/Orbitrap mass spectrometer. Anal Bioanal Chem 403:1279–1289
Althakafy JT, Kulsing C, Grace MR, Marriott PJ (2017) Liquid chromatography-quadrupole Orbitrap mass spectrometry method for selected pharmaceuticals in water samples. J Chromatogr A 1515:164–171
Hu Q, Noll RJ, Li H, Makarov A, Hardman M, Cooks RG (2005) The Orbitrap: a new mass spectrometer. J Mass Spectrom 40:430–443
Solliec M, Roy-Lachapelle A, Sauvé S (2015) Quantitative performance of liquid chromatography coupled to Q-Exactive high resolution mass spectrometry (HRMS) for the analysis of tetracyclines in a complex matrix. Anal Chim Acta 853:415–424
Fedorova G, Randak T, Lindberg RH, Grabic R (2013) Comparison of the quantitative performance of a Q-exactive high-resolution mass spectrometer with that of a triple quadrupole tandem mass spectrometer for the analysis of illicit drugs in wastewater. Rapid Commun Mass Spectrom 27:1751–1762
Rajski L, Gomez-Ramos Mdel M, Fernandez-Alba AR (2014) Large pesticide multiresidue screening method by liquid chromatography-Orbitrap mass spectrometry in full scan mode applied to fruit and vegetables. J Chromatogr A 1360:119–127
Zhang NR, Yu S, Tiller P, Yeh S, Mahan E, Emary WB (2009) Quantitation of small molecules using high-resolution accurate mass spectrometers—a different approach for analysis of biological samples. Rapid Commun Mass Spectrom 23:1085–1094
Kaufmann A (2014) Combining UHPLC and high-resolution MS: a viable approach for the analysis of complex samples? TrAC Trends Anal Chem 63:113–128
Del Mar Gomez-Ramos M, Rajski L, Heinzen H, Fernandez-Alba AR (2015) Liquid chromatography Orbitrap mass spectrometry with simultaneous full scan and tandem MS/MS for highly selective pesticide residue analysis. Anal Bioanal Chem 407:6317–6326
Acknowledgements
The authors are very grateful to the Victorian Institute of Forensic Medicine (VIFM) for providing drug standards and forensic cases for analysis. We also thank Thermo Fisher for provision of UHPLC with Q-Exactive MS facilities, together with the Xcalibur 3.0.63 and Trace Finder 3.0 software.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mokhtar, S.U., Kulsing, C., Althakafy, J.T. et al. Simultaneous Analysis of Drugs in Forensic Cases by Liquid Chromatography–High-Resolution Orbitrap Mass Spectrometry. Chromatographia 83, 53–64 (2020). https://doi.org/10.1007/s10337-019-03814-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03814-w