Abstract
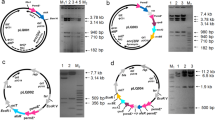
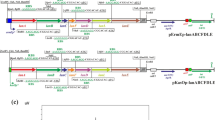
Some of the polyketide-derived bioactive compounds contain sugars attached to the aglycone core, and these sugars often impart specific biological activity to the molecule or enhance this activity. Mycinamicin II, a 16-member macrolide antibiotic produced by Micromonospora griseorubida A11725, contains a branched lactone and two different deoxyhexose sugars, d-desosamine and d-mycinose, at the C-5 and C-21 positions, respectively. The d-mycinose biosynthesis genes, mycCI, mycCII, mycD, mycE, mycF, mydH, and mydI, present in the M. griseorubida A11725 chromosome were introduced into pSET152 under the regulation of the promoter of the apramycin-resistance gene aac(3)IV. The resulting plasmid pSETmycinose was introduced into Micromonospora rosaria IFO13697 cells, which produce the 16-membered macrolide antibiotic rosamicin containing a branched lactone and d-desosamine at the C-5 position. Although the M. rosaria TPMA0001 transconjugant exhibited low rosamicin productivity, two new compounds, IZI and IZII, were detected in the ethylacetate extract from the culture broth. IZI was identified as a mycinosyl rosamicin derivative, 23-O-mycinosyl-20-deoxo-20-dihydro-12,13-deepoxyrosamicin (MW 741), which has previously been synthesized by a bioconversion technique. This is the first report on production of mycinosyl rosamicin-derivatives by a engineered biosynthesis approach. The integration site ΦC31attB was identified on M. rosaria IFO13697 chromosome, and the site lay within an ORF coding a pirin homolog protein. The pSETmycinose could be useful for stimulating the production of “unnatural” natural mycinosyl compounds by various actinomycete strains using the bacteriophage ΦC31 att/int system.






Similar content being viewed by others
References
Anzai Y, Ishii Y, Yoda Y, Kinoshita K, Kato F (2004) The targeted inactivation of polyketide synthase mycAV in the mycinamicin producer, Micromonospora griseorubida, and a complementation study. FEMS Microbiol Lett 238:315–320
Anzai Y, Kinoshita K, Seki T, Kato F (2004) Hybrid biosynthesis by targeted inactivation of polyketide synthases in the mycinamicin producer, Micronionospora griseorubida. J Antibiot 57:819–822
Anzai Y, Li S, Chaulagain MR, Kinoshita K, Kato F, Montgomery J, Sherman DH (2008) Functional analysis of MycCI and MycG, cytochrome P450 enzymes involved in biosynthesis of mycinamicin macrolide antibiotics. Chem Biol 15:950–959. doi:10.1016/j.chembiol.2008.07.014
Anzai Y, Saito N, Tanaka M, Kinoshita K, Koyama Y, Kato F (2003) Organization of the biosynthetic gene cluster for the polyketide macrolide mycinamicin in Micromonospora griseorubida. FEMS Microbiol Lett 218:135–141. doi:10.1111/j.1574-6968.2003.tb11509.x
Baltz RH, Seno ET (1981) Properties of Streptomyces fradiae mutants blocked in biosynthesis of the macrolide antibiotic tylosin. Antimicrob Agents Chemother 20:214–225
Choi SU, Lee CK, Hwang YI, Kinoshita H, Nihira T (2004) Intergeneric conjugal transfer of plasmid DNA from Escherichia coli to Kitasatospora setae, a bafilomycin B1 producer. Arch Microbiol 181:294–298. doi:10.1007/s00203-004-0654-8
Farnet CM, Staffa A, Yang X (2003) Genes and proteins for the biosynthesis of rosaramicin. World Intellectual Property Organization, Geneva. Pat WO 03010193-A:39
Flett F, Mersinias V, Smith CP (1997) High efficiency intergeneric conjugal transfer of plasmid DNA from Escherichia coli to methyl DNA-restricting streptomycetes. FEMS Microbiol Lett 155:223–229. doi:10.1111/j.1574-6968.1997.tb13882.x
Fouces R, Mellado E, Díez B, Barredo JL (1999) The tylosin biosynthetic cluster from Streptomyces fradiae: genetic organization of the left region. Microbiology 145:855–868
Funaishi K, Kawamura K, Satoh F, Hiramatsu M, Hagiwara M, Okanishi M (1990) New analogues of rosaramicin isolated from a Micromonospora strain. I. Taxonomy, fermentation, isolation and physico-chemical and biological properties. J Antibiot 43:938–947
Furumai T, Maezawa I, Matsuzawa N, Yano S, Yamaguchi T, Takeda K, Okuda T (1977) Macrolide antibiotics m-4365 produced by micromonospora. 1. Taxonomy, production, isolation, characterization and properties. J Antibiot 30:443–449
Ha HS, Hwang YI, Choi SU (2008) Application of conjugation using ϕC31 att/int system for Actinoplanes teichomyceticus, a producer of teicoplanin. Biotechnol Lett 30:1233–1238. doi:10.1007/s10529-008-9671-z
Hatano K, Higashide E, Shibata M (1976) Studies on juvenimicin, a new antibiotic. 1. Taxonomy, fermentation and antimicrobial properties. J Antibiot 29:1163–1170
HJung WS, Han AR, Hong JS, Park SR, Choi CY, Park JW, Yoon YJH (2007) Bioconversion of 12-, 14-, and 16-membered ring aglycones to glycosylated macrolides in an engineered strain of Streptomyces venezuelae. Appl Microbiol Biotechnol 76:1373–1381. doi:10.1007/s00253-007-1101-y
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hoopwood DA (2000) Practical Streptomyces genetics. John Innes Centre, Norwich
Kinoshita K, Imura Y, Takenaka S, Hayashi M (1989) Mycinamicins, new macrolide antibiotics. XI. Isolation and structure elucidation of a key intermediate in the biosynthesis of the mycinamicins, mycinamicin VIII. J Antibiot 42:1869–1872
Lee BK, Puar MS, Patel M, Bartner P, Lotvin J, Munayyer H, Waitz JA (1983) Multistep bioconversion of 20-deoxo-20-dihydro-12, 13-deepoxy-12, 13-dehydrorosaranolide to 22-hydroxy-23-O-mycinosyl-20-deoxo-20-dihydro-12, 13-deepoxyrosaramicin. J Antibiot 36:742–744
Olano C, Lomovskaya N, Fonstein L, Roll JT, Hutchinson CR (1999) A two-plasmid system for the glycosylation of polyketide antibiotics: bioconversion of epsilon-rhodomycinone to rhodomycin D. Chem Biol 6:845–855. doi:10.1016/S1074-5521(00)80004-6
Perez M, Lombo F, Baig I, Brana AF, Rohr J, Salas JA, Mendez C (2006) Combinatorial biosynthesis of antitumor deoxysugar pathways in Streptomyces griseus: Reconstitution of “unnatural natural gene clusters” for the biosynthesis of four 2, 6-d-dideoxyhexoses. Appl Environ Microbiol 72:6644–6652. doi:10.1128/AEM.01266-06
Rodriguez E, Hu Z, Ou S, Volchegursky Y, Hutchinson CR, McDaniel R (2003) Rapid engineering of polyketide overproduction by gene transfer to industrially optimized strains. J Ind Microbiol Biotechnol 30:480–488. doi:10.1007/s10295-003-0045-1
Rodriguez L, Aguirrezabalaga I, Allende N, Brana AF, Mendez C, Salas JA (2002) Engineering deoxysugar biosynthetic pathways from antibiotic-producing microorganisms: A tool to produce novel glycosylated bioactive compounds. Chem Biol 9:21–729. doi:10.1016/S1074-5521(02)00154-0
Salas JA, Mendez C (2007) Engineering the glycosylation of natural products in actinomycetes. Trends Microbiol 15:219–232. doi:10.1016/j.tim.2007.03.004
Sambrook J, Russell DW (2001) Molecular Cloning: A Laboratory Manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Satoi S, Muto N, Hayashi M, Fujii T, Otani M (1980) Mycinamicins, new macrolide antibiotics. 1. Taxonomy, production, isolation, characterization and properties. J Antibiot 33:364–376
Soo PC, Horng YT, Lai MJ, Wei JR, Hsieh SC, Chang YL, Tsai YH, Lai HC (2007) Pirin regulates pyruvate catabolism by interacting with the pyruvate dehydrogenase E1 subunit and modulating pyruvate dehydrogenase activity. J Bacteriol 189:109–118. doi:10.1128/JB.00710-06
Suzuki H, Takenaka S, Kinoshita K, Morohoshi T (1990) Biosynthesis of mycinamicins by a blocked mutant of Micromonospora griseorubida. J Antibiot 43:1508–1511
Tang L, McDaniel R (2001) Construction of desosamine containing polyketide libraries using a glycosyltransferase with broad substrate specificity. Chem Biol 8:547–555. doi:10.1016/S1074-5521(01)00032-1
Hosted TJ Jr, Wang T, Horan AC (2005) Characterization of the Micromonospora rosaria pMR2 plasmid and development of a high G + C codon optimized integrase for site-specific integration. Plasmid 54:249–258. doi:10.1016/j.plasmid.2005.05.004
Wagman GH, Weinstei MJ, Waitz JA, Testa RT, Oden EM, Murawski A, Marquez J (1972) New Micromonospora-produced macrolide antibiotic, rosamicin. J Antibiot 25:641–652
Acknowledgments
We thank H. Kieser (John Innes Centre) for E. coli strain ET12567/pUZ8002 and plasmid pSET152.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anzai, Y., Iizaka, Y., Li, W. et al. Production of rosamicin derivatives in Micromonospora rosaria by introduction of d-mycinose biosynthetic gene with ΦC31-derived integration vector pSET152. J Ind Microbiol Biotechnol 36, 1013–1021 (2009). https://doi.org/10.1007/s10295-009-0579-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-009-0579-y