Abstract
The quality of immune reconstitution (IR) is crucial for the outcome of patients who received allogeneic hematopoietic stem cell transplantation (allo-HSCT), and is closely connected with infection, relapse and graft-versus-host disease (GvHD) which are the most important causes for transplantation failure. However, the IR pattern in the early stage after allo-HSCT, particularly haploidentical (HID) HSCT, remains unclear. In this retrospective study, we examined the T cell reconstitution of patients within the initial 30 days (n = 173) and 100 days (n = 122) after allo-HSCT with myeloablative condition (MAC), of which > 70% were HID HSCT, to assess the influence of IR on the transplant outcomes. By comparing 78 patients with good IR (GIR) to 44 patients with poor IR (PIR), we observed that GIR was associated with lower risk for Epstein–Barr virus (EBV) reactivation and cytomegalovirus (CMV) reactivation, but had no significant impacts on the survival outcomes (i.e., overall survival, event-free survival) and cumulative incidences of GvHD. Importantly, we found lymphocyte reconstitution pattern at day 30 after allo-HSCT would be a surrogate for IR evaluated at day 100. In the Cox proportional hazard model, early reconstitution of CD4+, CD4+CD25+, CD4+CD45RO+, CD4+CD25+CD27low, and CD8+ T cells at day 30 was reversely correlated with risk of EBV reactivation. Finally, we constructed a predictive model for EBV reactivation with CD8+ and CD4+CD45RO+ T cell proportions of the training cohort (n = 102), which was validated with a validation cohort (n = 37). In summary, our study found that the quality of IR at day 30 had a predictive value for the risk of EBV reactivation, and might provide guidance for close monitoring for EBV reactivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative therapy for hematological malignancies. However, infection, relapse and graft-versus-host disease (GvHD) remain the major therapeutic challenges and affect the transplant outcomes. The quality of reconstitution of the donor-derived immune system in the recipient is of utmost importance for long-term survival after allo-HSCT [1,2,3]. Immune reconstitution (IR) would be completed in 2–5 years after allo-HSCT in a continuous and stepwise pattern, and this process would be affected by various factors [4]. Different kinds of immune cell subsets recover with different dynamics. After intensive conditioning, transplant recipients were in the “pre-engraftment phase” with prolonged neutropenia. Previous studies have confirmed that ATG_based prophylaxis are related to superior CD8+ T, γδ T, natural killer (NK) and NKT cell reconstitution, while the reconstitution of CD4+ T, regulatory T and B cell are faster in PTCy-based GvHD prophylaxis [5, 6] Neutrophil recovers within 14–30 days after graft infusion which defends against bacterial and fungal infections, which may be delayed after PTCy treatment, resulting more infections before day 100 [6]. The initial 100 days after transplantation are characterized by cellular immunodeficiencies due to a paucity of NK and T cells [7]. The compromised T cell reconstitution is primarily responsible for deleterious viral reactivations, including cytomegalovirus (CMV) and Epstein–Barr virus (EBV), as well as related viral end-organ diseases, rendering major reasons for morbidity and mortality for allo-HSCT [8].
Successful IR was defined as CD4+ T cells > 50 cells/μL in two consecutive measurements within 100 days after allo-HSCT [9, 10], which was associated with increased overall survival (OS) [9] and reduced non-relapse mortality (NRM) [10]. Besides, patients who received ATG treatment showed slower lymphocytes [11], CD4+ T, CD4+ CD25+ CD127− T (Treg) and CD4+ CD25− CD127+ T (Tconv) cell reconstitution [12] and higher rates of EBV reactivation [6], which indicated a potential association of EBV reactivation and CD4+ T subsets. However, the predictive value of CD4+ T cell recovery for the risk of viral reactivation is still weak [9, 10]. About 90% people in develo** countries had primary EBV and CMV infections during childhood and adolescence [19]. Interestingly, Itzykson R et al. revealed that the CMV serostatus in recipient was positively correlated with the proportions of HLA-DR+ activated (CD8+HLA-DR+) and of late effector memory CD8+ (CD8+CD45RA+CCR7−) T cells [20]. Besides, successful EBV-specific immune responses are characterized by effective cytotoxic CD8+ T cells and NK cells [21, 22]. Higher proportion of CD8+ T cells had been observed in patients with EBV reactivation [15, 23]. After EBV reactivation, a sustained low proportion of CD4+ T cells was persistent within one year [24]. These findings suggested that numerous kinds of CD4+ and CD8+ cells participated in immune responses after allo-HSCT. However, detailed studies concerning the IR pattern at early stage after allo-HSCT, and its underlying significance for transplantation-related complications are still lacking.
In the present study, we retrospectively investigated the quality of IR at day 30 and day 100 after allo-HSCT, respectively, and constructed a predictive model with proportions of CD8+ and CD4+CD45RO+ populations at day 30 to predict EBV reactivation. Our data provided a systematic characterization of early T-cell reconstitution and analyzed its relationship with EBV reactivation.
Patients and methods
Study cohorts
This is a retrospective analysis based on the allo-HSCT database of Shanghai Rui** Hospital. Consecutive adult patients receiving myeloablative allo-HSCT from April 2021 to April 2023 were screened, and the eligibility criteria were as follows: (1) age ≥ 16 years; (2) with a life expectancy ≥ 3 months; (3) receiving myeloablative condition; (4) during the first 3 months after allo-HSCT, the lymphocyte subsets were monitored at least once by flow cytometry. The last follow-up was July 30, 2023.
Transplantation procedures
The protocol for the preconditioning regimen, GvHD prophylaxis and treatment, and infection prophylaxis were the same as previously reported [25, 26]. In brief, calcineurin inhibitors with short-term methotrexate and mycophenolate mofetil were served as the backbone for the GvHD prophylaxis. Among all patients included in this analysis, anti-thymocyte globulin (ATG), posttransplant cyclophosphamide (PTCy), a combination of ATG and PTCy [27], and a combination of Cyclosporin A (CSA) and Methotrexate (MTX) were adapted for 37, 15, 118 and 3 patients, respectively. The stem cell sources were granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood stem cell grafts from haploidentical related donors (n = 134), HLA-matched related donors (n = 22), and HLA-matched unrelated donors (n = 17).
IR monitoring
After reaching neutrophil engraftment (> 500 cells/μL) following allo-HSCT, lymphocyte subsets were evaluated with flow cytometry at every week for up to 3 months. Cell acquisition was performed with a CantoII flow cytometer (BD Biosciences). All antibodies were titrated for optimum performance, and appropriate single-color compensation and fluorescence minus-one (FMO) controls were run. A time gate was initially drawn to ensure stable collection of samples. Cells in the “live” gate (Brilliant Violet 510-) were restricted by CD14- gate to remove monocytes and then by size (FSC) and granularity (SSC) to identify lymphocytes. Data were analyzed with FlowJo software, version 10.8.1.
Monitoring for infections
CMV and EBV reactivation were monitored weekly by assessing plasma CMV and EBV DNA through real-time quantitative polymerase chain reaction (RT-qPCR) up to 1 year after allo-HSCT. We defined CMV and EBV DNA viremia as the detection of any level of CMV and EBV DNA in plasma samples. The EBV reactivation was defined as more than 1 × 103 IU/mL EBV-DNA in plasma. CMV disease was diagnosed according to established criteria [28]. PTLD was defined by World Health Organization (WHO) classification of lymphoid neoplasms (2016 revision) [29]. The severity of infection was defined by Technical Manual of Procedures of Blood and Marrow Transplant Clinical Trials Network (version 3.0), consistent with previous study [30].
Machine learning
Classification and regression tree (CART) machine-based learning was applied for EBV reactivation dichotomized as a binary outcome. The candidate factors for subgroup identification were summarized in Supplemental Table 1. All the variables have been established to correlate with allo-HSCT outcomes. For the EBV reactivation prediction model, lymphocyte subsets routinely monitored at day 30 after allo-HSCT were chosen as the median time of EBV activation was 60 (range: 25–355) days in our patients.
Statistical analysis
Chi-square or Fisher’s exact test was used in different categorical variables between two groups. Continuous variables were compared by unpaired two-tailed Student’s t test (for two group comparisons) or a one-way ANOVA. OS and event-free survival (EFS) were estimated using Kaplan–Meier curves and compared using the log-rank (Mantel-Cox) test. Cumulative incidence (CI) was used to determine the probability of relapse, CMV infection, EBV infection, aGvHD and chronic GvHD (cGvHD), and death as a competing risk. As the excellent protection of letermovir for CMV reactivation, only patients (n = 77) who did not receive letermovir as CMV prophylaxis were included in the analysis of the CIs of CMV reactivation. The frequency of infection was calculated as the average times of infection per patient.
Univariate logistics regression model was used to determine the predictive value of lymphocyte subsets at day 30 after allo-HSCT for the IR cohort. A P-value < 0.05 was considered statistically significant. To quantify the prediction performance of the lymphocyte subsets for IR quality, receiver operating characteristic (ROC) curve with calculated area under curve (AUC) was plotted. Univariate Cox regression model was used to determine prognostic factors for the training cohort (n = 102). Multivariate Cox regression analysis was performed on the variables that were statistically significant in the univariate analysis. The nomogram model for the risk of EBV reactivation was constructed by the final Cox regression analysis. The calibration curve was plotted to measure the calibration of the risk model. To quantify the prediction performance of the risk model, C-index was calculated via bootstrap** validation (1,000 bootstrap resamples), and the ROC curve was drawn to determine the best threshold for distinguishing the risk of EBV reactivation. The baseline characteristics of training and validation cohorts are summarized in Supplemental Table 2. All analyses were performed by statistical software R version 4.2.
Results
Patients’ characteristics and outcomes related to IR
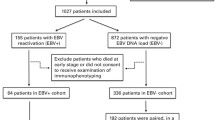
A total of 173 patients received MAC allo-HSCT with follow-up periods of more than 100 days were included in this study. One hundred and twenty-two patients were eligible for IR analysis, and 72.95% (89/122) of them received HID-HSCT. According to whether the patient achieved CD4+ T cells > 50 cells/μL in two consecutive measurements within 100 days after allo-HSCT [9], 78 patients were grouped as GIR and 44 patients were as PIR (Fig. 1). The median CD4+ T cell count for patients in GIR at day 30 was significantly higher than PIR (50 [1–465] vs. 4.5 [0–119] cells/μL, P < 0.0001) (Supplemental Fig. 1). Both groups were comparable in terms of most baseline transplant and disease characteristics, and the incidences of aGvHD and cGvHD. However, more patients in the GIR group received ATG as the GvHD prophylaxis (Table 1), which is consistent with previous studies [5, 6, 31].
Haploidentical transplantation was related to delayed CD3+ and CD4+ T cell reconstitution compared to matched transplantation since day 60 after allo-HSCT but did not affect the constitution of their subpopulations (Supplemental Fig. 2). Furthermore, we compared the IR of these two cohorts, and no statistical difference was observed (Supplemental Fig. 3A). The EBV reactivation rates of GIR and PIR patients in the haplo cohort and the matched cohort were comparable (Supplemental Fig. 3B). Except for the proportion of CD4+CD45RA+ T cells which recovered better in GIR patients of the matched cohort, no significant differences were found in the remaining subpopulations in either comparison group (Supplemental Fig. 3C and D).
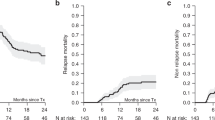
Compared to patients in GIR, patients in PIR suffered more frequently with grade 3 infections after day 100 (0.23 vs. 0.64, P = 0.0226) (Table 1). The CIs of CMV and EBV reactivation were much lower in patients from GIR group (62.2% vs. 100%, P = 0.0043; 25.2% vs. 55.7%, P = 0.0037) (Fig. 2A, B), as well as the incidences of CMV disease and PTLD (1.28% vs. 15.91% P = 0.0033; 0% vs. 6.82%, P = 0.0449) (Table 1). There were no statistically significant differences between the GIR and PIR groups in the CIs of acute/chronic GvHD and the survival outcomes (CIR, OS, EFS) (Fig. 2C–G).
Early lymphocyte recovery at day 30 reflects IR quality
Since the median times for reactivation of CMV and EBV were 41 (range: 10–110) days and 60 (range: 25–355) days in our cohort after allo-HSCT, we wondered whether the lymphocyte recovery pattern at early stage after transplantation (i.e., day 30) could discriminate patients with GIR and PIR. The consecutive recovery of CD3+, CD4+, CD8+, CD4+CD25+ (Treg), CD4+CD25+CD127low (Natural Treg, nTreg), and CD4+CD45RO+ T (CD4+ T memory) cells proportion were better in GIR group compared with their counterparts in PIR group (Supplemental Fig. 1). The differences had already appeared on day 30 after allo-HSCT (Table 2). The findings suggested that early lymphocyte recovery, especially CD4+ T-cell subpopulations at day 30 after allo-HSCT, may discriminate IR quality after allo-HSCT.
In univariate logistics analysis, we confirmed that higher proportions of CD3+, CD4+, CD4+CD25+, CD4+CD45RO+, CD4+CD28+, and CD8+ T subsets were associated with GIR (Fig. 3). Further, we examined the threshold of these subpopulations to distinguish IR status by ROC curve. Apart from the CD8+ T cell proportion, most of the other subsets had acceptable efficacy (AUC > 0.7). The most distinguishable T-cell compartments were the proportions of CD4+, CD4+CD28+, CD4+CD45RO+ and CD4+CD25+ (Supplemental Fig. 4), which were consistent with the result of univariant analysis.
Association of EBV reactivation and with the early reconstitution of lymphocytes. Univariate analysis (A) and multivariate analysis (B) using a Cox proportional hazard model. The outcome of EBV reactivation after allo-HSCT, with the main lymphocyte subsets at day 30 day. (C) Venn diagram of lymphocyte subsets at day 30 after allo-HSCT affecting IR and EBV reactivation
CD8+ and CD4+CD45RO+ reconstitution predicts EBV reactivation
Next, we tested whether the early T-cell reconstitution would predict the risk of EBV reactivation in all 173 patients. Compared to patients without EBV reactivation, the reconstitution of TCR(ɑ/β), TCR(γ/δ), CD3+, CD3+HLA-DR+, CD4+, CD4+CD25+, CD4+CD25+CD127low, CD4+HLA-DR+, CD4+CD45RO+, CD4+CD45RA−CD27+, CD8+ and CD8+CD28+ T cells at day 30 of patients with EBV reactivation was inferior (Supplemental Fig. 5). Univariate analysis confirmed that higher proportions of these subsets were associated with a decreased risk of EBV reactivation (Fig. 4A). Decreased proportions of CD4+CD25+, CD4+CD25+CD127low and CD4+CD45RO+ T cells at day 30 were risk factors for EBV reactivation (Fig. 4A). In multivariate analysis, a higher CD4+CD45RO+ proportion at day 30 after allo-HSCT was associated with a lower risk of EBV reactivation (HR: 0.928, 95%CI [0.879–0.980], P = 0.008) (Fig. 4B).
Venn diagram showed that lymphocyte subsets discriminating IR were shared with those in relation with EBV activation at day 30 after allo-HSCT. Higher proportions of CD45RO + , TCR(ɑ/β), CD3+, CD4+, CD4+CD25+, CD4+CD45RO+, CD4+CD28+, and CD8+ were positively associated with GIR and served as protective factors of EBV reactivation (Fig. 4C). These results showed that EBV reactivation was correlated with early T-cell IR after allo-HSCT.
Construction of a predictive model for EBV reactivation
To address the importance of these subsets influencing EBV reactivation, we utilized CART analysis to calculate the weight of early lymphocyte subsets and clinical characteristics in association with EBV reactivation. Among all variables included in the machine learning, the proportion of CD4+CD45RO+ T cells at day 30 after allo-HSCT had the highest Gini index (Fig. 5A). When the percent of CD4+CD45RO+ cells was greater than 7.25%, the risk for EBV reactivation was significantly reduced (Fig. 5B). These results were consistent with the results of Cox regression analysis described above (Fig. 4A, B), suggesting that CD4+CD45RO+ T cells may be essential in controlling EBV reactivation.
As multivariate analysis demonstrated that higher CD4+CD45RO+ proportion at day 30 after allo-HSCT was associated with lower risk of EBV reactivation and CD8+ T-cells were crucial in defense of EBV reactivation [32, 33], consistent with our univariant analysis (Fig. 4A), we developed a predictive model for EBV reactivation within 1 year after allo-HSCT by the Cox regression analysis with training cohort. Two key predictors, the proportions of CD8+ and CD4+CD45RO+ cells of total lymphocytes, were identified and presented in the nomogram (Fig. 6A). The calibration curve demonstrated that the nomogram had good concordance to predict the risk of EBV reactivation in this cohort (Fig. 6B). The C-index of the nomogram was 0.706, suggesting a good discriminative ability for this risk model. The AUC of ROC curve for this model was 0.772, which showed a high predictive value (Fig. 6C). Besides, using the predictive model, we divided patients in the training cohort into high-risk and low-risk groups (cut-off predictive probability > 0.309, which had higher specificity). The CIs of EBV reactivation of these two groups were 54.5% and 23.2% (P < 0.0001) (Fig. 6D), respectively.
Early reconstitution of CD8+ and CD4+CD45RO+ T cells predict EBV reactivation within 1 year after allo-HSCT. (A) Nomogram model for EBV reactivation prediction of allo-HSCT patients with CD8+ and CD4+CD45RO+ T cells proportions at day 30. (B) Calibration plot of the predictive model. (C) ROC analysis of predicted probability for EBV reactivation. CIs of EBV reactivation of different patients in training cohort (IR cohort) (D) and validation cohort (E) based on the predicted probability of EBV reactivation. High risk: predicted probability > 0.309; Low risk: predicted probability ≤ 0.309
To further verify the predictive value of the model, we screened 37 patients with measurements of CD8+ and CD4+CD45RO+ T cells at day 30 from 51 patients without complete IR data, as the validation cohort. According to the EBV reactivation predictive model, there were 23 and 14 patients each in the high-risk group and the low-risk group. The CIs of EBV reactivation of these two groups were 74.6% and 29.3% (P = 0.02) (Fig. 6E), respectively. These results indicated that early CD8+ and CD4+CD45RO+ T cell recovery at day 30 could predict the occurrence of EBV reactivation after allo-HSCT.
Discussion
In this study, we retrospectively analyzed the association of immune cell recovery at day 30 and IR quality of 122 patients who received myeloablative allo-HSCT. As previous reported, CD4+ T cells reconstitution could predict OS in pediatric HSCT patients [9, 34], and better IR was associated with less viral reactivation [9]. In the present study, we confirmed that early recovery of T cell subsets at day 30 reflected IR quality in a cohort with more than 70% HID HSCT. And successful reconstitution of CD4+ T cells within 100 days was associated with lower CI of EBV reactivation. Several variables contribute to the increased risk of EBV reactivation after allo-HSCT. HLA mismatch is the most important risk factor for EBV reactivation after allo-HSCT [35,36,37]. Therefore, patients with HID HSCT would have a high risk for EBV reactivation. To note, we set the EBV-DNAemia (> 1000 IU/ml) as the diagnostic criterion for EBV reactivation according to the previous study [38]. As EBV-DNAemia accumulated, the risk of EBV-PTLD rose rapidly [38]. 27.40% (20/73) of patients with EBV reactivation in our cohort required rituximab intervention. Accurate prediction and close monitoring for EBV activation are warranted. Thus, we evaluated the T cell reconstitution at day 30 after allo-HSCT, and found that the lymphocyte reconstitution at day 30 effectively predicted EBV reactivation, which would be ahead of conventional IR evaluation timepoint (100 days post-transplantation).
Adaptive immunity is the core determinant for EBV prevention [39,40,41]. Several factors have a role in affecting immune recovery after allo-HSCT, spanning donor age, donor gender, intensive chemotherapy, GvHD prophylaxis, and conditioning regimens [42,43,44,45]. T cell recovery after allo-HSCT differs across individuals, and immune-monitoring might help to predict the risk of EBV reactivation shortly after allo-HSCT. ROC analysis revealed that the proportion of CD4+ and its subpopulation could distinguish GIR from PIR, while PIR patients are associated with higher incidence of EBV reactivation and PTLD, suggested that early CD4+ reconstitution may predict EBV infection post-transplantation. Our findings confirmed that impaired CD4+ T cell recovery was correlated with EBV reactivation and PTLD, which is consistent with previous studies [46, 47].
During primary EBV infection in healthy individuals, T cell numbers in peripheral blood were increased dramatically [39]. EBV-specific immune cells differentiated into memory CD4+ (including CD45RA−CD45RO+CCR7− effector memory [EM] and CD45RA−CD45RO+CCR7+ central memory [CM] ~ 0.1%) and CD8+ (2–5%) T cells after infection [48]. For allo-HSCT recipients, early recovery of donor-derived EBV-specific T cells within 60 days provided prophylactic effects against EBV-related diseases [49]. The early recovery of the T cells relies on peripheral expansion of memory T cells, and CD8+ T cells reconstitute earlier than CD4+ T cells in early T-cell reconstitution. In the present study, CD8+ and CD4+CD45RO+ T cells within 30 days was reversely associated with EBV reactivation. Thus, our data suggested that CD8+ and CD4+CD45RO+ T cells after allo-HSCT provided a protection against EBV reactivation, possibly by driving early recovery of EBV-specific T cells. Furthermore, in the clinical setting, the criterion of the invention against EBV reactivation after allo-HSCT is not well established. Our data provided a promising method for risk-stratification of EBV reactivation, which might assist the judgment for early intervention.
Tregs is a subpopulation of CD4+ T cells with the function of suppressing immune responses and maintaining self-tolerance [50]. In our data, Tregs (CD4+CD25+ and CD4+CD25+CD127low T cells) at day 30 after allo-HSCT were independent protective factors of EBV reactivation, which is consistent with previous studies that poor CD4+CD25+ T cell recovery at day 30 after allo-HSCT was associated with prolonged CMV and EBV duration [51, 52]. Taking the high inflammatory status of the early period after allo-HSCT into consideration, Tregs could have a compensatory increase and reflect high cytotoxic activity of effector T cells [53]. These findings suggested that a more careful evaluation of Tregs function in CMV/EBV reactivation is necessary, especially in the early period after allo-HSCT. Besides, there are many studies focus on the differential impact of CMV on outcome/immune reconstitution depending on CMV kinetics and higher CMV load is related to poor IR and clinical outcomes [5, 54, 55]. However, CMV reactivation after allo-HSCT is significantly limited in our cohort (data not shown) in the letermovir era, which is the reason that we're focused on predicting EBV activation with IR.
Our study has several limitations due to its retrospective design, small sample size, and short follow-up duration. Ongoing follow-up observation of survival in all patients is needed to verify the long-term effects of IR in HSCT patients. And the predictive model should be further evaluated by external cohorts. Thus, larger multicenter retrospective studies or prospective research endeavors are warranted.
In summary, our data suggested that early lymphocytes recovery, especially the CD4+ T cell and its subsets, were correlated with the quality of IR. We developed a prognostic nomogram for EBV reactivation based on the proportion of CD4+CD45RO+ and CD8+ T cells at day 30 after allo-HSCT, which may help to surveil the risk of EBV reactivation in early stages and intervene promptly.
References
Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115(19):3861–8.
Ando T, et al. Impact of graft sources on immune reconstitution and survival outcomes following allogeneic stem cell transplantation. Blood Adv. 2020;4(2):408–19.
Ogonek J, et al. Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol. 2016;7:507.
De Koning C, Nierkens S, Boelens JJ. Strategies before, during, and after hematopoietic cell transplantation to improve T-cell immune reconstitution. Blood. 2016;128(23):2607–15.
Leserer S, et al. Time series clustering of T cell subsets dissects heterogeneity in immune reconstitution and clinical outcomes among MUD-HCT patients receiving ATG or PTCy. Front Immunol. 2023;14:1082727.
Massoud R, et al. Comparison of immune reconstitution between anti-T-lymphocyte globulin and posttransplant cyclophosphamide as acute graft-versus-host disease prophylaxis in allogeneic myeloablative peripheral blood stem cell transplantation. Haematologica. 2022;107(4):857–67.
Fry TJ, Mackall CL. Immune reconstitution following hematopoietic progenitor cell transplantation: challenges for the future. Bone Marrow Transplant. 2005;35(Suppl 1):S53–7.
Roddie C, Peggs KS. Immunotherapy for transplantation-associated viral infections. J Clin Investig. 2017;127(7):2513–22.
Admiraal R, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. 2015;2(5):e194–203.
de Koning C, et al. CD4+ T-cell reconstitution predicts survival outcomes after acute graft-versus-host-disease: a dual-center validation. Blood. 2021;137(6):848–55.
Turki AT, et al. Optimizing anti-T-lymphocyte globulin dosing to improve long-term outcome after unrelated hematopoietic cell transplantation for hematologic malignancies. Am J Transplant. 2020;20(3):677–88.
Gooptu M, et al. Effect of antihuman T lymphocyte globulin on immune recovery after myeloablative allogeneic stem cell transplantation with matched unrelated donors: analysis of immune reconstitution in a double-blind randomized controlled trial. Biol Blood Marrow Transplant. 2018;24(11):2216–23.
**ong G, et al. Epstein-Barr virus (EBV) infection in Chinese children: a retrospective study of age-specific prevalence. PLoS ONE. 2014;9(6): e99857.
Peric Z, et al. Features of Epstein-Barr Virus (EBV) reactivation after reduced intensity conditioning allogeneic hematopoietic stem cell transplantation. Leukemia. 2011;25(6):932–8.
Alexandersson A, et al. Viral infections and immune reconstitution interaction after pediatric allogenic hematopoietic stem cell transplantation. Infect Dis. 2019;51(10):772–8.
Heslop HE. How I treat EBV lymphoproliferation. Blood. 2009;114(19):4002–8.
Khandelwal P, et al. Peripheral blood CD38 bright CD8+ effector memory T cells predict acute graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(7):1215–22.
Bejanyan N, et al. Delayed immune reconstitution after allogeneic transplantation increases the risks of mortality and chronic GVHD. Blood Adv. 2018;2(8):909–22.
Camargo JF, et al. Deep functional immunophenoty** predicts risk of cytomegalovirus reactivation after hematopoietic cell transplantation. Blood. 2019;133(8):867–77.
Itzykson R, et al. Cytomegalovirus shapes long-term immune reconstitution after allogeneic stem cell transplantation. Haematologica. 2015;100(1):114–23.
Martinez OM, Krams SM. The immune response to Epstein Barr virus and implications for posttransplant lymphoproliferative disorder. Transplantation. 2017;101(9):2009–16.
Hatton O, et al. NKG2A-expressing natural killer cells dominate the response to autologous lymphoblastoid cells infected with Epstein-Barr virus. Front Immunol. 2016;7:607.
Illiaquer M, et al. Impact of stem cell graft on early viral infections and immune reconstitution after allogeneic transplantation in adults. J Clin Virol. 2017;93:30–6.
Zhou X, et al. Clinical value of plasma and peripheral blood mononuclear cells Epstein-Barr Virus DNA dynamics on prognosis of allogeneic stem cell transplantation. Front Cell Infect Microbiol. 2022;12: 980113.
Zhang Y, et al. Allogeneic hematopoietic stem cells transplantation improves the survival of intermediate-risk acute myeloid leukemia patients aged less than 60 years. Ann Hematol. 2019;98(4):997–1007.
Jiang J-L, et al. Low incidence of relapse with a moderate conditioning regimen of fludarabine, busulfan, and melphalan for patients with myeloid malignancies: a single-center analysis of 100 patients. Transplant Cell Ther. 2023;29(8):512.e1-512.e8.
Duléry R, Brissot E, Mohty M. Combining post-transplant cyclophosphamide with antithymocyte globulin for graft-versus-host disease prophylaxis in hematological malignancies. Blood Rev. 2023;2023:101080.
Ljungman P, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64(1):87–91.
Tamaru J-I. 2016 revision of the WHO classification of lymphoid neoplasms [Rinsho Ketsueki]. Jpn J Clin Hematol. 2017;58(10):2188–93.
Bolaños-Meade J, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med. 2023;388(25):2338–48.
Chang Y-J, Zhao X-Y, Huang X-J. Immune reconstitution after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(4):440–9.
Meij P, et al. Impaired recovery of Epstein-Barr virus (EBV)–specific CD8+ T lymphocytes after partially T-depleted allogeneic stem cell transplantation may identify patients at very high risk for progressive EBV reactivation and lymphoproliferative disease. Blood. 2003;101(11):4290–7.
Vietzen H, et al. HLA-E-restricted immune responses are crucial for the control of EBV infections and the prevention of PTLD. Blood. 2023;141(13):1560–73.
Bartelink IH, et al. Immune reconstitution kinetics as an early predictor for mortality using various hematopoietic stem cell sources in children. Biol Blood Marrow Transplant. 2013;19(2):305–13.
Fan S, et al. Machine learning algorithm as a prognostic tool for Epstein-Barr virus reactivation after haploidentical hematopoietic stem cell transplantation. Blood Sci. 2023;5(1):51–9.
Ru Y, et al. Epstein-Barr virus reactivation after allogeneic hematopoietic stem cell transplantation: multifactorial impact on transplant outcomes. Bone Marrow Transplant. 2020;55(9):1754–62.
Styczynski J, et al. Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: sixth European conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101(7):803–11.
Lindsay J, et al. Dynamics of Epstein-Barr virus on post-transplant lymphoproliferative disorders after antithymocyte globulin-conditioned allogeneic hematopoietic cell transplant. Transplant Infect Dis. 2021;23(5): e13719.
Taylor GS, et al. The immunology of Epstein-Barr virus-induced disease. Annu Rev Immunol. 2015;33:787–821.
Palendira U, Rickinson AB. Primary immunodeficiencies and the control of Epstein-Barr virus infection. Ann N Y Acad Sci. 2015;1356:22–44.
Damania B, Münz C. Immunodeficiencies that predispose to pathologies by human oncogenic γ-herpesviruses. FEMS Microbiol Rev. 2019;43(2):181–92.
Gournay V, et al. Immune landscape after allo-HSCT: TIGIT- and CD161-expressing CD4 T cells are associated with subsequent leukemia relapse. Blood. 2022;140(11):1305–21.
Zhou Y, et al. Differential analysis of immune reconstitution after allogeneic hematopoietic stem cell transplantation in children with Wiskott-Aldrich syndrome and chronic granulomatous disease. Front Immunol. 2023;14:1202772.
Mouton W, et al. Distinct immune reconstitution profiles captured by immune functional assays at 6 months post allogeneic hematopoietic stem cell transplantation. Transplant Cell Ther. 2023;29(2):94e1–13.
Fan ZY, et al. CMV infection combined with acute GVHD associated with poor CD8+ T-cell immune reconstitution and poor prognosis post-HLA-matched allo-HSCT. Clin Exp Immunol. 2022;208(3):332–9.
Patriarca F, et al. Prognostic factors and outcome of Epstein-Barr virus DNAemia in high-risk recipients of allogeneic stem cell transplantation treated with preemptive rituximab. Transplant Infect Dis. 2013;15(3):259–67.
Landgren O, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113(20):4992–5001.
Tangye SG, Latour S. Primary immunodeficiencies reveal the molecular requirements for effective host defense against EBV infection. Blood. 2020;135(9):644–55.
Zhou L, Lu D-P. Immune reconstitution of HLA-A*0201/BMLF1- and HLA-A*1101/LMP2-specific Epstein Barr virus cytotoxic T lymphocytes within 90 days after haploidentical hematopoietic stem cell transplantation. Virol J. 2019;16(1):19.
Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6(4):331–7.
Zhou J-R, et al. Co-reactivation of cytomegalovirus and epstein-barr virus was associated with poor prognosis after allogeneic stem cell transplantation. Front Immunol. 2020;11: 620891.
Ngoma AM, et al. Impaired regulatory T cell reconstitution in patients with acute graft-versus-host disease and cytomegalovirus infection after allogeneic bone marrow transplantation. Int J Hematol. 2012;95(1):86–94.
Di Ianni M, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–8.
Duke ER, et al. CMV viral load kinetics as surrogate endpoints after allogeneic transplantation. J Clin Investig. 2021;131(1):e133960.
Hill JA, et al. Kinetics of double-stranded DNA viremia after allogeneic hematopoietic cell transplantation. Clin Infect Dis. 2018;66(3):368–75.
Acknowledgements
We thank Shanghai Rui** Hospital for the allo-HSCT database and all those who contributed to this study.
Funding
This work was supported by the National Natural Science Foundation of China (81770124, 82170206, 82200156), the National Key Research and Development Program of China (2022YFC2502600), Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant (20171909), Shanghai Municipal Health Commission Project of Disciplines of Excellence (20234Z0002), Youth Project of Shanghai Municipal Health Commission (20214Y0187).
Author information
Authors and Affiliations
Contributions
JH and ZP performed the analyses, collected clinical data and wrote the manuscript. LW, ZZ, CJ, and JH collected samples, treated patients, and interpreted the data. GC and TY proposed and designed the study, interpreted the data, wrote the manuscript, and oversaw the project.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests in relation to the work described.
Ethical approval
The study was approved by the institutional review board of Rui** hospital (Approved Number: 2021-173) and was conducted in accordance with the Declaration of Helsinki.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, J., Pan, Z., Wang, L. et al. Early T-cell reconstitution predicts risk of EBV reactivation after allogeneic hematopoietic stem cell transplantation. Clin Exp Med 24, 22 (2024). https://doi.org/10.1007/s10238-023-01270-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10238-023-01270-3