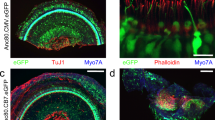
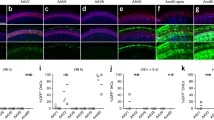
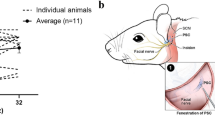
Abstract
Sensorineural losses of hearing and vestibular sensation due to hair cell dysfunction are among the most common disabilities. Recent preclinical research demonstrates that treatment of the inner ear with a variety of compounds, including gene therapy agents, may elicit regeneration and/or repair of hair cells in animals exposed to ototoxic medications or other insults to the inner ear. Delivery of gene therapy may also offer a means for treatment of hereditary hearing loss. However, injection of a fluid volume sufficient to deliver an adequate dose of a pharmacologic agent could, in theory, cause inner ear trauma that compromises functional outcome. The primary goal of the present study was to assess that risk in rhesus monkeys, which closely approximates humans with regard to middle and inner ear anatomy. Secondary goals were to identify the best delivery route into the primate ear from among two common surgical approaches (i.e., via an oval window stapedotomy and via the round window) and to determine the relative volumes of rhesus, rodent, and human labyrinths for extrapolation of results to other species. We measured hearing and vestibular functions before and 2, 4, and 8 weeks after unilateral injection of phosphate-buffered saline vehicle (PBSV) into the perilymphatic space of normal rhesus monkeys at volumes sufficient to deliver an atoh1 gene therapy vector. To isolate effects of injection, PBSV without vector was used. Assays included behavioral observation, auditory brainstem responses, distortion product otoacoustic emissions, and scleral coil measurement of vestibulo-ocular reflexes during whole-body rotation in darkness. Three groups (N = 3 each) were studied. Group A received a 10 μL transmastoid/trans-stapes injection via a laser stapedotomy. Group B received a 10 μL transmastoid/trans-round window injection. Group C received a 30 μL transmastoid/trans-round window injection. We also measured inner ear fluid space volume via 3D reconstruction of computed tomography (CT) images of adult C57BL6 mouse, rat, rhesus macaque, and human temporal bones (N = 3 each). Injection was well tolerated by all animals, with eight of nine exhibiting no signs of disequilibrium and one animal exhibiting transient disequilibrium that resolved spontaneously by 24 h after surgery. Physiologic results at the final, 8-week post-injection measurement showed that injection was well tolerated. Compared to its pretreatment values, no treated ear’s ABR threshold had worsened by more than 5 dB at any stimulus frequency; distortion product otoacoustic emissions remained detectable above the noise floor for every treated ear (mean, SD and maximum deviation from baseline: −1.3, 9.0, and −18 dB, respectively); and no animal exhibited a reduction of more than 3 % in vestibulo-ocular reflex gain during high-acceleration, whole-body, passive yaw rotations in darkness toward the treated side. All control ears and all operated ears with definite histologic evidence of injection through the intended site showed similar findings, with intact hair cells in all five inner ear sensory epithelia and intact auditory/vestibular neurons. The relative volumes of mouse, rat, rhesus, and human inner ears as measured by CT were (mean ± SD) 2.5 ± 0.1, 5.5 ± 0.4, 59.4 ± 4.7 and 191.1 ± 4.7 μL. These results indicate that injection of PBSV at volumes sufficient for gene therapy delivery can be accomplished without destruction of inner ear structures required for hearing and vestibular sensation.








Similar content being viewed by others
References
Akil O, Rouse SL, Chan DK, Lustig LR (2015) Surgical method for virally mediated gene delivery to the mouse inner ear through the round window membrane. J Vis Exp 16(97). doi:10.3791/52187
Borenstein JT (2011) Intracochlear drug delivery systems. Expert Opin Drug Deliv. 8(9):1161–1174
Brown RF, Hullar TE, Cadieux JH, Chole RA (2010) Residual hearing preservation after pediatric cochlear implantation. Otol Neurotol. 31(8):1221–1216
Bruce IA, Bates JE, Melling C, Mawman D, Green KM (2011) Hearing preservation via a cochleostomy approach and deep insertion of a standard length cochlear implant electrode. Otol Neurotol. 32(9):1444–14447
Buckingham RA, Valvassori GE (2001) Inner ear fluid volumes and the resolving power of magnetic resonance imaging: can it differentiate endolymphatic structures? Ann Otol Rhinol Laryngol 110(2):113–117
Calabrese DR, Hullar TE (2006) Planar relationships of the semicircular canals in two strains of mice. JARO. 2006;7(2):151–159. Epub 2006 Apr 22. PubMed PMID: 16718609; PubMed Central PMCID: PMC2504575.
Carey JP and Della Santina CC, Vestibular Physiology, in Cummings Otolaryngology–Head & Neck Surgery, 5th Edn (Editor P. W. Flint), Elsevier. 2009.
Dai C, Fridman GY, Della Santina CC (2011a) Effects of vestibular prosthesis electrode implantation and stimulation on hearing in rhesus monkeys. Hear res 277(1–2):204–210
Dai C, Fridman GY, Davidovics NS, Chiang B, Ahn JH, Della Santina CC (2011b) Restoration of 3D vestibular sensation in rhesus monkeys using a multichannel vestibular prosthesis. Hear res 281(1–2):74–83
Dai C, Fridman GY, Chiang B, Davidovics NS, Melvin T, Cullen KE, Della Santina CC (2011c) Cross-axis adaptation improves 3D vestibulo-ocular reflex alignment during chronic stimulation via a head-mounted multichannel vestibular prosthesis. Exp Brain res 210:595–606
Ekdale EG (2013) Comparative anatomy of the bony labyrinth (inner ear) of placental mammals. PLoS One 8(6):e66624
Erixon E, Köbler S, Rask-Andersen H (2012) Cochlear implantation and hearing preservation: results in 21 consecutively operated patients using the round window approach. ActaOtolaryngol 132(9):923–931
Fetter M, Zee DS(1988) Recovery from unilateral labyrinthectomy in rhesus monkey. J Neurophysiol 59(2):370-393
Havenith S, Lammers MJ, Tange RA, Trabalzini F, della Volpe A, van der Heijden GJ, Grolman W (2013) Hearing preservation surgery: cochleostomy or round window approach? A systematic review. Otol Neurotol. 34(4):667–674
Helbig S, Rajan GP, Stöver T, Lockley M, Kuthubutheen J, Green KM (2013) Hearing preservation after cochlear re-implantation. Otol Neurotol 34(1):61–65
Hill T, Lewicki P (2007) Statistics: methods and applications. Dell, Tulsa
Jung JY, Avenarius MR, Adamsky S, Alpert E, Feinstein E, Raphael Y (2013) siRNA targeting Hes5 augments hair cell regeneration in aminoglycoside-damaged mouse utricle. Mol Ther 21:834–841
Klickstein L (2013), CGF166 atonal gene therapy for hearing loss & vestibular dysfunction: Review of NIH OBA protocol #1310–1260. Accessed April 1, 2015 at: http://osp.od.nih.gov/sites/default/files/1_1260_CGF166_Klickstein.pdf
Kraft S, Hsu C, Brough DE, Staecker H (2013) Atoh1 induces auditory hair cell recovery in mice after ototoxic injury. Laryngoscope 123(4):992–999
Lasky RE, Snodgrass EB, Laughlin NK, Hecox KE (1995) Distortion product otoacoustic emissions in Macaca mulatta and humans. Hear res 89:35–51
Martin GK, Lonsbury-Martin BL, Probst R, Coats AC (1988) Spontaneous otoacoustic emissions in a nonhuman primate. I Basic Features and Relations to Other Emissions Hear res 33:49–68
McCall AA, Swan EE, Borenstein JT, Sewell WF, Kujawa SG, McKenna MJ (2010) Drug delivery for treatment of inner ear disease: current state of knowledge. Ear Hear 31:156–165
Migliaccio AA, Schubert MC, Jiradejvong P, Lasker DM, Clendaniel RA, Minor LB (2004) The three-dimensional vestibulo-ocular reflex evoked by high-acceleration rotations in the squirrel monkey. Exp Brain res 159:433–446
Minor LB, Lasker DM, Backous DD, Hullar TE (1999) Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. I Normal Responses J Neurophysiol 82:1254–1270
Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge AS (2013) Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 77:58–69
Park JY, Clark WW, Coticchia JM, Esselman GH, Fredrickson JM (1995) Distortion product otoacoustic emissions in rhesus (Macaca mulatta) monkey ears: normative findings. Hear res 86:147–162
Praetorius M, Brough DE, Hsu C, Plinkert PK, Pfannenstiel SC, Staecker H (2009) Adenoviral vectors for improved gene delivery to the inner ear. Hear res 248(1–2):31–38
Robinson DA (1963) A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10:137–145
Sadeghi SG, Minor LB, Cullen KE (2006) Dynamics of the horizontal vestibuloocular reflex after unilateral labyrinthectomy: response to high frequency, high acceleration, and high velocity rotations. Exp Brain Res 175(3):471-484
Sadeghi SG, Minor LB, Cullen KE (2007) Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol 97(2):1503–1514
Salt AN, Hale SA, Plonkte SK (2006) Perilymph sampling from the cochlear apex: a reliable method to obtain higher purity perilymph samples from scala tympani. J Neurosci Methods 153(1):121–129
Schubert MC, Migliaccio AA, Della Santina CC (2006) Dynamic visual acuity during passive head thrusts in canal planes. J Assoc res Otolaryngol 7(4):329–338
Shinomori Y, Spack DS, Jones DD, Kimura RS (2001) Volumetric and dimensional analysis of the guinea pig inner ear. Ann Otol Rhinol Laryngol 110(1):91–98
Staecker H, Praetorius M, Brough DE (2011) Development of gene therapy for inner ear disease: using bilateral vestibular hypofunction as a vehicle for translational research. Hear res 276(1–2):44–51
Staecker H, Rodgers B (2013) Developments in delivery of medications for inner ear disease. Expert Opin Drug Deliv 10(5):639–650
Staecker H, Schlecker C, Kraft S, Praetorius M, Hsu C, Brough DE (2014) Optimizing atoh1-induced vestibular hair cell regeneration. Laryngoscope 124(Suppl 5):S1–S12
Straumann D, Zee DS, Solomon D, Lasker AG, Roberts DC (1995) Transient torsion during and after saccades. Vis res 35:3321–3334
Stuart A, Stenstrom R, Tompkins C, Vandenhoff S (1991) Test-retest variability in audiometric threshold with supraaural and insert earphones among children and adults. Audiology 30(2):82–90
Sun DQ, Ward BK, Semenov YR, Carey JP, Della Santina CC (2014) Bilateral vestibular deficiency: Quality of life and economic implications. JAMA Otolaryngol Head Neck Surg 140(6):527–534
Torre P, Mattison JA, Fowler CG, Lane MA, Roth GS, Ingram DK (2004) Assessment of auditory function in rhesus monkeys (Macaca mulatta): effects of age and calorie restriction. Neurobiol Aging 25(7):945–954
Tweed D, Cadera W, Vilis T (1990) Computing three-dimensional eye position quaternions and eye velocity from search coil signals. Vis res 30(1):97–110
Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, Sgro BE, O'Leary SJ, Shepherd RK, Richardson RT (2010) Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther 18(6):1111–1122. doi:10.1038/mt.2010.28
Zou J, Zhang W, Poe D, Zhang Y, Ramadan UA, Pyykkö I (2010) Differential passage of gadolinium through the mouse inner ear barriers evaluated with 4.7T MRI. Hear Res 259(1–2):36–43
Acknowledgements
This research was supported by a contract to JHU from the Novartis Institutes for Biomedical Research. We thank Alicia White for assistance with ABR and DPOAE measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, C., Lehar, M., Sun, D.Q. et al. Rhesus Cochlear and Vestibular Functions Are Preserved After Inner Ear Injection of Saline Volume Sufficient for Gene Therapy Delivery. JARO 18, 601–617 (2017). https://doi.org/10.1007/s10162-017-0628-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-017-0628-6