ABSTRACT
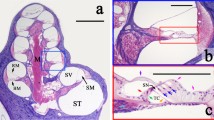
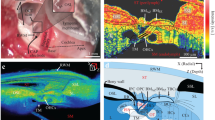
The cells in the organ of Corti are highly organized, with a precise 3D microstructure hypothesized to be important for cochlear function. Here we provide quantitative data on the mouse organ of Corti cytoarchitecture, as determined using a new technique that combines the imaging capabilities of two-photon microscopy with the autofluorescent cell membranes of the genetically modified mTmG mouse. This combination allowed us to perform in situ imaging on freshly excised tissue, thus minimizing any physical distortions to the tissue that extraction from the cochlea and chemical fixation and staining might have caused. 3D image stacks of the organ of Corti were obtained from base to apex in the cochlear duct, from which 3D lengths and relative angles for inner and outer hair cells, Deiters’ cells, phalangeal processes, and inner and outer pillars were measured. In addition, intercellular distances, diameters, and stereocilia shapes were obtained. An important feature of this study is the quantitative reporting of the longitudinal tilts of the outer hair cells towards the base of the cochlea, the tilt of phalangeal processes towards the apex, and Deiters’ cells that collectively form a Y-shaped building block that is thought to give rise to the lattice-like organization of the organ of Corti. The variations of this Y-shaped element along the cochlear duct and between the rows of outer hair cells are shown with the third row morphologically different from the other rows, and their potential importance for the cochlear amplifier is discussed.










Similar content being viewed by others
Abbreviations
- BM:
-
Basilar membrane
- DC:
-
Deiters’ cell
- IP:
-
Inner pillar
- IHC:
-
Inner hair cell
- OHC:
-
Outer hair cell
- OoC:
-
Organ of Corti
- OP:
-
Outer pillar
- PhP:
-
Phalangeal process
- RL:
-
Reticular lamina
- TM:
-
Tectorial membrane
- ToC:
-
Tunnel of Corti
REFERENCES
Angelborg C, Engstrom H (1972) Supporting elements in the organ of Corti. I. Fibrillar structures in the supporting cells of the organ of Corti of mammals. Acta Otolaryngol. Suppl 73:49–60
Berens P (2009) CircStat: a Matlab toolbox for circular statistics. J Stat Softw 31–10
Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y (1985) Evoked mechanical responses of isolated cochlear outer hair cells. Science (New York, N.Y.) 227(4683):194–6
Buytaert JAN, Johnson SB, Dierick M, Salih WHM, Santi PA (2013) MicroCT versus sTSLIM 3D imaging of the mouse cochlea. J Histochem Cytoc 61:382–395
Cooper NP, Rhode WS (1997) Mechanical responses to two-tone distortion products in the apical and basal turns of the mammalian cochlea. J Neurophysiol 78:261–270
Dannhof BJ, Roth B, Bruns V (1991) Length of hair cells as a measure of frequency representation in the mammalian inner ear? Die Naturwissenschaften 78(12):570–573
Drobizhev M, Tillo S, Makarov NS, Hughes TE, Rebane A (2009) Absolute two-photon absorption spectra and two-photon brightness of orange and red fluorescent proteins. J Phys Chem B 113(4):855–859
Drobizhev M, Makarov NS, Tillo S, Hughes TE, Rebane A (2011) Two-photon absorption properties of fluorescent proteins. Nat Methods 8(5):393–399
Gao SS, **a A, Yuan T et al (2011) Quantitative imaging of cochlear soft tissues in wild-type and hearing-impaired transgenic mice by spectral domain optical coherence tomography. Opt Express 19:15415–15428
Geisler D, Sang C (1995) A cochlear model using feed-forward outer-hair-cell forces. Hear Res 86:132–146
Hartman BH, Reh T, Bermingham-McDonogh O (2010) Notch signaling specifies prosensory domains via lateral induction in the develo** mammalian inner ear. PNAS 107:15792–15797
Karavitaki KD (2002). Measurements and models of electrically-evoked motion in the gerbil organ of Corti. Ph.D. Thesis, MIT, Cambridge
Keiler S, Richter C-P (2001) Cochlear dimensions obtained in hemicochleae of four different strains of mice: CBA/CaJ, 129/CD1, 129/SvEv and C57BL/6J. Hear Res 162:91–104
Kolmer W (1913) Studien am Labyrinth von Insectivoren, Sitzungsberichte Akademie der Wissenschaften, Abteilung III, pp. 29–57
Liberman MC, Dodds LW, Pierce S (1990) Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J Comp Neurol
Lim DJ (1986) Functional structure of the organ of Corti: a review. Hear Res 22:117–146
Liu CC, Gao SS, Yuan T, Steele C, Puria S, Oghalai JS (2011) Biophysical mechanisms underlying outer hair cell loss associated with a shortened tectorial membrane. JARO 12(5):577–594
Maison SF, Adams JC, Liberman MC (2003) Olivocochlear innervation in the mouse: immunocytochemical maps, crossed versus uncrossed contributions, and transmitter colocalization. J Comp Neurol 455:406–416
Mikaelian D, Ruben RJ (1965) Development of hearing in the normal CBA-J mouse: Correlation of physiological observations with behavioral responses and with cochlear anatomy. Acta Oto-Laryngologica 59.2–6:451–461.
Müller M, von Hünerbein K, Hoidis S, Smolders JWT (2005) A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res 202:63–73
Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L (2007) A global double-fluorescent Cre reporter mouse. Genesis (New York, NY: 2000) 45(9):593–605
Nam JH (2014) Microstructures in the Organ of Corti help outer hair cells form traveling waves along the cochlear coil. Biophys J 106(11):2426–2433
Neely ST, Kim DO (1983) An active cochlear model showing sharp tuning and high sensitivity. Hear Res 9:123–130
Parsa A, Webster P, Kalinec F (2012) Deiters cells tread a narrow path—the Deiters cells–basilar membrane junction. Hear Res 290:13–20
Puria S, Hartman B, Kim J, Oghalai JS, Ricci AJ, Liberman MC (2011) Three-dimensional imaging of the mouse organ of Corti cytoarchitecture for mechanical modeling. AIP Conf. Proc. pp 356–362
Ren T, He W (2011) Measurement of basilar membrane, reticular lamina, and tectorial membrane vibrations in the intact mouse cochlea. AIP Conf. Proc 1403, 423
Retzius G (1884) Das Gehörorgan der Wirbeltiere, vol. 2. Samson & Wallin, Stockholm
Shera CA, Guinan JJ (2003) Stimulus-frequency-emission group delay: a test of coherent reflection filtering and a window on cochlear tuning. J Acoust Soc Am 113:2762
Shera CA, Zweig (1993) Order from chaos: resolving the paradox of periodicity in evoked otoacoustic emission. Biophys Hair Cell Sens Syst 54–63
Slepecky NB (1996) Structure of the mammalian cochlea, chapter 2 in the cochlea. In: Dallos PJ, Popper AN, Fay RR (Eds.) (Springer handbook of auditory research)
So PT (2002) Two-photon fluorescence light microscopy. Encyclopedia of Life Sciences, Nature Publishing Group 1–5
Soons J, Dirckx JJJ, Puria S, and Steele CR (2014) Basilar membrane and reticular lamina motion in a multi-scale finite element model of the mouse cochlea. In: Mechanics of Hearing 2014, AIP proceedings (submitted)
Spicer SS, Schulte BA (1994) Differences along the place-frequency map in the structure of supporting cells in the gerbil cochlea. Hear Res 79:161–177
Steele CR, Baker G, Tolomeo J, Zetes D (1993) Electro-mechanical models of the outer hair cell. In: Duifhuis H, Horst JW, van Dijk P, van Netten SM (eds) Biophysics of hair cell sensory systems. World Scientific, Singapore, pp 207–215
Szalai R, Epp B, Champneys A, Homer M (2011) On time-delayed and feed-forward transmission line models of the cochlea. J Mech Mat Struct 6(1):557–568
Tiedemann H (1970) A new approach to theory of hearing. Acta Otolaryngol Suppl 277:1–50
Voldřich L (1983) Experimental and topographic morphology in cochlear mechanics. In: de Boer E, Viergever MA (eds) Mechanics of hearing. Delft University Press, Delft, pp 163–167
Wen B, Boahen K (2003) A linear cochlear model with active bi-directional coupling. The 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC 2003), pp. 2013–2016
Yoon Y-J, Puria S, Steele CR (2009) A cochlear model using the time-averaged Lagrangian and the push-pull mechanism in the organ of Corti. J Mech Mater Struct 4(5):977–986
Yoon Y-J, Steele CR, Puria S (2011) Feed-forward and feed-backward amplification model from cochlear cytoarchitecture: an interspecies comparison. Biophys J 100:1–10. doi:10.1016/j.bpj.2010.11.039
Zetes DE, Tolomeo J, Holley MC (2012) Structure and mechanics of supporting cells in the guinea pig organ of Corti. PLoS One 7:e49338
Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P (2000) Prestin is the motor protein of cochlear outer hair cells. Nature 405(6783):149–155. doi:10.1038/35012009
ACKNOWLEDGMENTS
The authors thank M. C. Liberman for helpful discussions and J. Buytaert for sharing the μCT scans of the mouse cochlea. This research was funded by NIH NIDCD grants R01 DC007910 (to SP), R01 DC003896 (to AJR), Core Grant P30 44992, and shared instrumentation grant NIH S10RR027267-01 as well as Research Foundation Flanders (FWO) and Belgian American Educational Foundation (BAEF) grants (to JS).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 77 kb)
Rights and permissions
About this article
Cite this article
Soons, J.A.M., Ricci, A.J., Steele, C.R. et al. Cytoarchitecture of the Mouse Organ of Corti from Base to Apex, Determined Using In Situ Two-Photon Imaging. JARO 16, 47–66 (2015). https://doi.org/10.1007/s10162-014-0497-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-014-0497-1