Abstract
Background
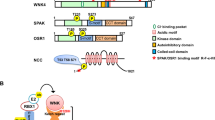
Pseudohypoaldosteronism type II (PHAII) is a hereditary hypertensive disease caused by mutations in four genes: WNK1, WNK4, Kelch-like3 (KLHL3), and cullin3 (CUL3). Recently, it was revealed that CUL3–KLHL3 E3 ligase complex ubiquitinates WNK1 and WNK4, leading to their degradation, and that a common pathogenesis of PHAII is defective WNK degradation due to CUL3–KLHL3 E3 ligase complex impairment. PHAII-causing CUL3 mutations mediate exon9 skip**, producing a CUL3 protein with a 57-amino acid deletion (Δ403–459). However, the pathogenic effects of KLHL3, an adaptor protein that links WNKs with CUL3, in PHAII caused by CUL3 mutation remain unclear.
Methods
To clarify detailed pathophysiological mechanisms underlying PHAII caused by CUL3 mutation in vivo, we generated and analyzed knock-in mice carrying the same CUL3 exon9 deletion (CUL3WT/Δex9) as that reported in PHAII patients.
Results
CUL3WT/Δex9 mice exhibited a PHAII-like phenotype. Interestingly, we confirmed markedly decreased KLHL3 expression in CUL3WT/Δex9 mice by confirming the true KLHL3 band in vivo. However, the expression of other KLHL family proteins, such as KLHL2, was comparable between WT and mutant mice.
Conclusion
KLHL3 expression was decreased in CUL3WT/Δex9 mice. However, expression levels of other KLHL family proteins were comparable between the wild-type and mutant mice. These findings indicate that the decreased abundance of KLHL3 is a specific phenomenon caused by mutant CUL3 (Δexon9). Our findings would improve our understanding of the pathogenesis of PHAII caused by CUL3 mutation in vivo.



Similar content being viewed by others
References
Gordon RD. Syndrome of hypertension and hyperkalemia with normal glomerular filtration rate. Hypertension. 1986;8:93–102.
Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–12.
Bergaya S, Faure S, Baudrie V, Rio M, Escoubet B, Bonnin P, Henrion D, Loirand G, Achard JM, Jeunemaitre X, Hadchouel J. WNK1 regulates vasoconstriction and blood pressure response to α 1-adrenergic stimulation in mice. Hypertension. 2011;58:439–45.
Yang C, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide- sensitive Na–Cl cotransport. J Clin Invest. 2003;111:1039–45.
Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride–coupled cotransporters via the STE20-related Kinases. J Biol Chem. 2005;280:42685–93.
Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482:98–102.
Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet. 2012;44:456–60.
Ji AX, Privé GG. Crystal structure of KLHL3 in complex with cullin3. PLoS One. 2013;8:1–10.
Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. CellReports. 2013;3:858 – 68.
Wu G, Peng J, Bin. Disease-causing mutations in KLHL3 impair its effect on WNK4 degradation. FEBS Lett. 2013;587:1717–22.
Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci. 2013;110:7838–43.
Ohta A, Schumacher FR, Mehellou Y, Johnson C, Knebel A, Macartney TJ, Wood NT, Alessi DR, Kurz T. The CUL3–KLHL3 E3 ligase complex mutated in Gordon’s hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J. 2013;451:111–22.
Schumacher FR, Siew K, Zhang J, Johnson C, Wood N, Cleary SE, Al Maskari RS, Ferryman JT, Hardege I, Figg NL, Enchev R, Knebel A, O’Shaughnessy KM, Kurz T. Characterisation of the Cullin-3 mutation that causes a severe form of familial hypertension and hyperkalaemia. EMBO Mol Med. 2015;7:1285–306.
Ferdaus MZ, Miller LN, Agbor LN, Saritas T, Singer JD, Sigmund CD, McCormick JA. Mutant Cullin 3 causes familial hyperkalemic hypertension via dominant effects. JCI Insight. 2017;2:1–16.
McCormick JA, Yang CL, Zhang C, Davidge B, Blankenstein KI, Terker AS, Yarbrough B, Meermeier NP, Park HJ, McCully B, West M, Borschewski A, Himmerkus N, Bleich M, Bachmann S, Mutig K, Argaiz ER, Gamba G, Singer JD, Ellison DH. Hyperkalemic hypertension—associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest. 2014;124:4723–36.
Tsuji S, Yamashita M, Unishi G, Takewa R, Kimata T, Isobe K, Chiga M, Uchida S, Kaneko K. A young child with pseudohypoaldosteronism type II by a mutation of Cullin 3. BMC Nephrology. 2013;14:1.
Chen H-Y, Liu C-C, Chen R-H. Cul3-KLHL20 ubiquitin ligase: physiological functions, stress responses, and disease implications. Cell Div. 2016;11:5.
Sohara E, Rai T, Yang SS, Uchida K, Nitta K, Horita S, Ohno M, Harada A, Sasaki S, Uchida S. Pathogenesis and treatment of autosomal-dominant nephrogenic diabetes insipidus caused by an aquaporin 2 mutation. Proc Natl Acad Sci. 2006;103:14217–22.
Sakai K, Miyazaki JI. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun. 1997;237:318–24.
Chiga M, Rafiqi FH, Alessi DR, Sohara E, Ohta A, Rai T, Sasaki S, Uchida S. Phenotypes of pseudohypoaldosteronism type II caused by the WNK4 D561A missense mutation are dependent on the WNK-OSR1/SPAK kinase cascade. J Cell Sci. 2011;124:1391–5.
Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4D561A/+ Knockin mouse model. Cell Metab. 2007;5:331–44.
Kasagi Y, Takahashi D, Aida T, Nishida H, Nomura N, Zeniya M, Mori T, Sasaki E, Ando F, Rai T, Uchida S, Sohara E. Impaired degradation of medullary WNK4 in the kidneys of KLHL2 knockout mice. Biochem Biophys Res Commun. 2017;487:368–74.
Takahashi D, Mori T, Nomura N, Hossain Khan MZ, Araki Y, Zeniya M, Sohara E, Rai T, Sasaki S, Uchida S. WNK4 is the major WNK positively regulating NCC in the mouse kidney. Biosci Rep. 2014;34:195–206.
Ohta A, Rai T, Yui N, Chiga M, Sen Yang S, Lin SH, Sohara E, Sasaki S, Uchida S. Targeted disruption of the Wnk4 gene decreases phosphorylation of Na–Cl cotransporter, increases Na excretion and lowers blood pressure. Hum Mol Genet. 2009;18:3978–86.
Sohara E, Rai T, Yang SS, Ohta A, Naito S, Chiga M, Nomura N, Lin SH, Vandewalle A, Ohta E, Sasaki S, Uchida S. Acute insulin stimulation induces phosphorylation of the Na–Cl cotransporter in cultured distal mpkDCT cells and mouse kidney. PLoS One. 2011;6:e24277.
Sandberg MB, Riquier ADM, Pihakaski-Maunsbach K, McDonough A, Maunsbach AB. ANG II provokes acute trafficking of distal tubule Na+–Cl(-) cotransporter to apical membrane. Am J Physiol Renal Physiol. 2007;293:F662–9.
Isobe K, Mori T, Asano T, Kawaguchi H, Nonoyama S, Kumagai N, Kamada F, Morimoto T, Hayashi M, Sohara E, Rai T, Sasaki S, Uchida S. Development of enzyme-linked immunosorbent assays for urinary thiazide-sensitive Na–Cl cotransporter measurement. Am J Physiol Renal Physiol. 2013;305:F1374-81.
Cleveland DW, Yamanaka K, Bomont P. Gigaxonin controls vimentin organization through a tubulin chaperone-independent pathway. Hum Mol Genet. 2009;18:1384–94.
Araki Y, Rai T, Sohara E, Mori T, Inoue Y, Isobe K, Kikuchi E, Ohta A, Sasaki S, Uchida S. Generation and analysis of knock-in mice carrying pseudohypoaldosteronism type II-causing mutations in the. Biol Open. 2015;4:1509–17.
Singer JD, Gurian-West M, Clurman B, Roberts JM. Cullin-3 targets cyclin E for ubiquitination and controls S phase in mammalian cells. Genes Dev. 1999;13:2375–87.
Sasaki E, Susa K, Mori T, Isobe K, Araki Y, Inoue Y, Yoshizaki Y, Ando F, Mori Y, Mandai S, Zeniya M, Takahashi D, Nomura N, Rai T, Uchida S, Sohara E. KLHL3 knockout mice reveal the physiological role of KLHL3 and the pathophysiology of pseudohypoaldosteronism type II. Mol Cell Biol. 2017;37(7):e00508–e16.
Takahashi D, Mori T, Wakabayashi M, Mori Y, Susa K, Zeniya M, Sohara E, Rai T, Sasaki S, Uchida S. KLHL2 interacts with and ubiquitinates WNK kinases. Biochem Biophys Res Commun. 2013;437:457–62.
Kansanen E, Kuosmanen SM, Leinonen H, Levonenn AL. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–9.
Bomont P, Cavalier L, Blondeau F, Ben Hamida C, Belal S, Tazir M, Demir E, Topaloglu H, Korinthenberg R, Tüysüz B, Landrieu P, Hentati F, Koenig M. The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nat Genet. 2000;26:370–4.
Gross V, Luft FC. Exercising restraint in measuring blood pressure in conscious mice. Hypertension. 2003;41:879–81.
Acknowledgements
We thank C. Iijima and M. Chiga for help in the experiments. This work is supported by the Grants-in-Aid for Scientific Research (S) from the Japanese Society for the Promotion of Science (Grant No. 25221306–00), Grants-in-Aid for Scientific Research (B) from the Japanese Society for the Promotion of Science (Grant No. 16H05314), Grant-in-Aid for Challenging Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yoshida, S., Araki, Y., Mori, T. et al. Decreased KLHL3 expression is involved in the pathogenesis of pseudohypoaldosteronism type II caused by cullin 3 mutation in vivo. Clin Exp Nephrol 22, 1251–1257 (2018). https://doi.org/10.1007/s10157-018-1593-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-018-1593-z