Abstract
Background
Lynch-like syndrome (LLS) has recently been proposed as a third type of microsatellite instability (MSI) tumor after Lynch syndrome (LS) and sporadic MSI colorectal cancer (CRC) without either a germline variant of mismatch repair (MMR) genes or hypermethylation of the MLH1 gene. The present study aimed to clarify and compare the clinicopathological characteristics of LLS with those of the other MSI CRC subtypes.
Methods
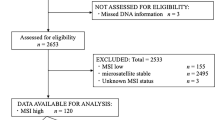
In total, 2634 consecutive patients with CRC who underwent surgical resection and subsequently received universal tumor screening (UTS), including MSI analysis were enrolled between January 2008 and November 2019. Genetic testing was performed in patients suspected of having Lynch syndrome.
Results
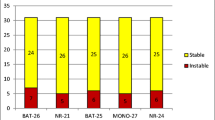
UTS of the cohort found 146 patients with MSI CRC (5.5%). Of these, excluding sporadic MSI CRC, 30 (1.1%) had a diagnosis of LS, and 19 (0.7%) had no germline pathogenic variants of the MMR gene. The CRC type in the latter group was identified as LLS. LLS occurred significantly more often in young patients, was left-sided, involved a KRAS variant and BRAF wild-type, and had a higher concordance rate with the Revised Bethesda Guidelines than sporadic MSI CRC. No significant differences were observed in terms of the clinicopathological factors between LLS and LS-associated MSI CRC; however, LLS had a lower frequency of LS-related neoplasms compared with LS.
Conclusions
Distinguishing clinically between LS and LLS was challenging, but the incidence of neoplasms was higher in LS than in LLS, suggesting the need for different screening and surveillance methods for the two subtypes.


Similar content being viewed by others
References
Vogelstein B, Fearon ER, Hamilton SR et al (1988) Genetic alterations during colorectal-tumor development. N Engl J Med 319:525–532
Miyaki M, Seki M, Okamoto M et al (1990) Genetic changes and histopathological types in colorectal tumors from patients with familial adenomatous polyposis. Cancer Res 50:7166–7173
Herman JG, Umar A, Polyak K et al (1998) Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA 95:6870–6875
Esteller M, Levine R, Baylin SB et al (1998) MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene 17:2413–2417
Kambara T, Simms LA, Whitehall VL et al (2004) BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut 53:1137–1144
Yamaguchi T, Iijima T, Mori T et al (2006) Accumulation profile of frameshift mutations during development and progression of colorectal cancer from patients with hereditary nonpolyposis colorectal cancer. Dis Colon Rectum 49:399–406
Rodriguez-Soler M, Perez-Carbonell L, Guarinos C et al (2013) Risk of cancer in cases of suspected lynch syndrome without germline mutation. Gastroenterology 144:926–932 (e921; quiz e913–924)
Kang SY, Park CK, Chang DK et al (2015) Lynch-like syndrome: characterization and comparison with EPCAM deletion carriers. Int J Cancer 136:1568–1578
Mensenkamp AR, Vogelaar IP, van Zelst-Stams WA et al (2014) Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gastroenterology 146:643-646.e648
Chika N, Eguchi H, Kumamoto K et al (2017) Prevalence of Lynch syndrome and Lynch-like syndrome among patients with colorectal cancer in a Japanese hospital-based population. Jpn J Clin Oncol 47:108–117
Provenzale D, Gupta S, Ahnen DJ et al (2016) Genetic/familial high-risk assessment: colorectal version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 14:1010–1030
Kang YJ, Killen J, Caruana M et al (2020) The predicted impact and cost-effectiveness of systematic testing of people with incident colorectal cancer for Lynch syndrome. Med J Aust 212:72–81
Ladabaum U, Wang G, Terdiman J et al (2011) Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann Intern Med 155:69–79
Win AK, Jenkins MA, Dowty JG et al (2017) Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev 26:404–412
Fujita M, Liu X, Iwasaki Y et al (2022) Population-based Screening for Hereditary Colorectal Cancer Variants in Japan. Clin Gastroenterol Hepatol 20:2132-2141.e2139
Golubicki M, Diaz-Gay M, Bonjoch L et al (2021) Comprehensive genomic characterization of fifteen early-onset lynch-like syndrome colorectal cancers. Cancers (Basel) 13:1259
Xu HX, Zhu P, Zheng YY et al (2020) Molecular screening and clinicopathologic characteristics of Lynch-like syndrome in a Chinese colorectal cancer cohort. Am J Cancer Res 10:3920–3934
Hampel H, Frankel WL, Martin E et al (2005) Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 352:1851–1860
Golubicki M, Bonjoch L, Acuna-Ochoa JG, et al (2020) Germline biallelic Mcm8 variants are associated with early-onset Lynch-like syndrome. JCI Insight 5
**cola RM, Clark JR, Carroll T et al (2019) Implication of DNA repair genes in Lynch-like syndrome. Fam Cancer 18:331–342
Antelo M, Golubicki M, Roca E et al (2019) Lynch-like syndrome is as frequent as Lynch syndrome in early-onset nonfamilial nonpolyposis colorectal cancer. Int J Cancer 145:705–713
Mas-Moya J, Dudley B, Brand RE et al (2015) Clinicopathological comparison of colorectal and endometrial carcinomas in patients with Lynch-like syndrome versus patients with Lynch syndrome. Hum Pathol 46:1616–1625
Overbeek LI, Kets CM, Hebeda KM et al (2007) Patients with an unexplained microsatellite instable tumour have a low risk of familial cancer. Br J Cancer 96:1605–1612
Xu Y, Huang Z, Li C et al (2020) Comparison of molecular, clinicopathological, and pedigree differences between lynch-like and lynch syndromes. Front Genet 11:991
Venderbosch S, Nagtegaal ID, Maughan TS et al (2014) Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 20:5322–5330
Bucksch K, Zachariae S, Aretz S et al (2020) Cancer risks in Lynch syndrome, Lynch-like syndrome, and familial colorectal cancer type X: a prospective cohort study. BMC Cancer 20:460
Pico MD, Sanchez-Heras AB, Castillejo A et al (2020) Risk of cancer in family members of patients with lynch-like syndrome. Cancers (Basel) 12:2225
Pearlman R, Haraldsdottir S, de la Chapelle A et al (2019) Clinical characteristics of patients with colorectal cancer with double somatic mismatch repair mutations compared with Lynch syndrome. J Med Genet 56:462–470
Acknowledgements
The authors thank all the patients and their families and Mr. James R. Valera for his assistance with editing this manuscript.
Funding
This work was funded by Japan Society for the Promotion of Science (Grant nos. 21K07187 ,18K07314, 21K16437).
Author information
Authors and Affiliations
Contributions
Sakiko Nakamori: Acquisition of data, analysis and interpretation of data, drafting the article, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Misato Takao, Akinari Takao, Soichiro Natsume, Daisiuke Nakano, Kazushige Kawai, Takuhiko Inokuchi, Makiko Urushibara, Shin-ichiro Horiguchi, and Hideyuki Ishida: Acquisition of data, critically revising the article, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Takeru Iijima and Ekumi Kojika: Analysis and interpretation of data, critically revising the article, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Tatsuro Yamaguchi: Conception and design, acquisition of date, drafting the article, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflict of interest.
Ethics statement
Approval of the research protocol by an Institutional Reviewer Board: The present study was approved by the ethical review board at Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital.
Informed consent
N/A.
Registry and the Registration No. of the study/trial
Approval number: 612, 1202, 1433, and 2001.
Animal studies
N/A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Nakamori, S., Takao, M., Takao, A. et al. Clinicopathological characteristics of Lynch-like syndrome. Int J Clin Oncol 29, 944–952 (2024). https://doi.org/10.1007/s10147-024-02527-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02527-x