Abstract
Background
Tisagenlecleucel, an autologous CD19-directed T-cell immunotherapy, can induce a durable response in adult patients with relapsed/refractory (r/r) B-cell lymphoma.
Methods
To elucidate the outcome of chimeric antigen receptor (CAR) T-cell therapy in Japanese, we retrospectively analyzed the outcomes of 89 patients who received tisagenlecleucel for r/r diffuse large B-cell lymphoma (n = 71) or transformed follicular lymphoma (n = 18).
Results
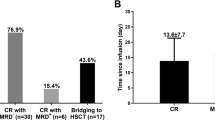
With a median follow-up of 6.6-months, 65 (73.0%) patients achieved a clinical response. The overall survival (OS) and event-free survival (EFS) rates at 12 months were 67.0% and 46.3%, respectively. Overall, 80 patients (89.9%) had cytokine release syndrome (CRS), and 6 patients (6.7%) had a grade ≥ 3 event. ICANS occurred in 5 patients (5.6%); only 1 patient had grade 4 ICANS. Representative infectious events of any grade were cytomegalovirus viremia, bacteremia and sepsis. The most common other adverse events were ALT elevation, AST elevation, diarrhea, edema, and creatinine elevation. No treatment-related mortality was observed. A Sub-analysis showed that a high metabolic tumor volume (MTV; ≥ 80 ml) and stable disease /progressive disease before tisagenlecleucel infusion were both significantly associated with a poor EFS and OS in a multivariate analysis (P < 0.05). Notably, the combination of these 2 factors efficiently stratified the prognosis of these patients (HR 6.87 [95% CI 2.4–19.65; P < 0.05] into a high-risk group).
Conclusion
We report the first real-world data on tisagenlecleucel for r/r B-cell lymphoma in Japan. Tisagenlecleucel is feasible and effective, even in late line treatment. In addition, our results support a new algorithm for predicting the outcomes of tisagenlecleucel.




Similar content being viewed by others
References
Sehn LH, Gascoyne RD (2015) Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood 125(1):22–32
Tilly H, Morschhauser F, Sehn LH et al (2022) Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med 386(4):351–363
Crump M, Neelapu SS, Farooq U et al (2017) Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 130(16):1800–1808
Van Den Neste E, Schmitz N, Mounier N et al (2016) Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant 51(1):51–57
Schuster SJ, Tam CS, Borchmann P et al (2021) Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 22(10):1403–1415
Pasquini MC, Hu ZH, Curran K et al (2020) Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv 4(21):5414–5424
Goto H, Makita S, Kato K et al (2020) Efficacy and safety of tisagenlecleucel in Japanese adult patients with relapsed/refractory diffuse large B-cell lymphoma. Int J Clin Oncol 25(9):1736–1743
Schuster SJ, Bishop MR, Tam CS et al (2019) Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 380(1):45–56
Vercellino L, Di Blasi R, Kanoun S et al (2020) Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv 4(22):5607–5615
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32(27):3059–3068
Jain T, Bar M, Kansagra AJ et al (2019) Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive b cell non-Hodgkin lymphoma: an expert panel opinion from the american society for transplantation and cellular therapy. Biol Blood Marrow Transplant 25(12):2305–2321
Senjo H, Hirata K, Izumiyama K et al (2020) High metabolic heterogeneity on baseline 18FDG-PET/CT scan as a poor prognostic factor for newly diagnosed diffuse large B-cell lymphoma. Blood Adv 4(10):2286–2296
Hirata K, Kobayashi K, Wong KP et al (2014) A semi-automated technique determining the liver standardized uptake value reference for tumor delineation in FDG PET-CT. PLoS ONE 9(8):e105682
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48(3):452–458
Hirayama AV, Gauthier J, Hay KA et al (2019) The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood 133(17):1876–1887
Hay KA, Gauthier J, Hirayama AV et al (2019) Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood 133(15):1652–1663
Sarris AH, Majlis A, Dimopoulos MA et al (1995) Rising serum lactate dehydrogenase often caused by granulocyte-or Granulocyte-macrophage colony stimulating factor and not tumor progression in patients with lymphoma or myeloma. Leuk Lymphoma 17(5–6):473–477
Bakker J, de Backer D, Hernandez G (2016) Lactate-guided resuscitation saves lives: we are not sure. Intensive Care Med 42(3):472–474
Tabbara IA (1992) Hemolytic anemias. Diagnosis and management. Med Clin North Am 76(3):649–668
Hong R, Yin TS, E, Wang L, et al (2021) Tumor burden measured by 18F-FDG PET/CT in predicting efficacy and adverse effects of chimeric antigen receptor T-Cell therapy in non-Hodgkin lymphoma. Front Oncol 11:713577
Lee DW, Santomasso BD, Locke FL et al (2019) ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 25(4):625–638
Arcangeli S, Bove C, Mezzanotte C et al (2022) CAR T cell manufacturing from naive/stem memory T lymphocytes enhances antitumor responses while curtailing cytokine release syndrome. J Clin Invest 132(12):e150807
Giavridis T, van der Stegen SJC, Eyquem J et al (2018) CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 24(6):731–738
**ao X, Huang S, Chen S et al (2021) Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J Exp Clin Cancer Res 40(1):367
Acknowledgements
We thank all of the patients and their families who participated in this study. We are also grateful to Fumina Kusaka for her help in our research. We thank Japan Medical Communication for providing editorial assistance for this manuscript.
Author information
Authors and Affiliations
Contributions
H.G., T.K., N.F., K.K., Y.O., N.F., T.Y. and T.T. designed the study; H.G., T.K., N.F., K.K., Y.O., N.F., T.Y., K.T., H.K., S.Y., M.O., M.S., and K.O. provided assembled data; H.G., T.K., N.F., K.K., Y.O., N.F., T.Y., K.T., H.K., S.Y., M.S., and K.O. enrolled and treated patients; H.G., H.S., I.Y., and K.H. analyzed and interpreted the data; All authors participated in writing the manuscript, and provided feedback throughout the development process.
Corresponding author
Ethics declarations
Conflict of interest
H.G. has received honoraria from Bristol-Myers Squibb, Eisai, Janssen, Chugai, Novartis, SymBio, Kyowa-Kirin, Astellas, MSD and Daiichi-Sankyo; and has received research grants from Bristol-Myers Squibb and Kyowa-Kirin; and has received research funding from Kyowa-Kirin, AbbVie and Chugai. T.K. has received honoraria from Novartis, Otsuka, Bristol-Myers Squibb, Chugai, SymBio and Asahi Kasei. N.F. has received honoraria from Novartis, Chugai, Daiichi-Sankyo, Nippon Shinyaku, Janssen, Pfizer and Astellas. K.K. has received honoraria from Novartis, Chugai and Kyowa-Kirin; and has received research funding from Chugai, Takeda, Kyowa-Kirin, AbbVie, Novartis, Eisai, Janssen, Bristol-Myers Squibb, Ono and Daiichi-Sankyo. Y.O. has received honoraria from Novartis, Pfizer, Janssen, AbbVie, Chugai, Bristol-Myers Squibb, Amgen, Kyowa-Kirin, SymBio, Astellas, MSD, Meiji Seika; and has received research funding from Novartis, Pfizer, Janssen and Takara Bio. N.F. has received honoraria from AstraZeneca, Bristol-Myers Squibb, Chugai, CLS Behring, Dainippon Sumitomo, Eisai, Janssen, Kyowa-Kirin, Nippon Shinyaku, Novartis, Ono, Otsuka, Sanofi, SymBio, Takeda and Zenyaku; and has received research funding from Bayer, Chugai, Celgene, Genmab and Incyte. K.O. has received honoraria from Novartis, AbbVie, Astellas, Janssen and Nippon Shinyaku; and has received research funding from Bristol-Myers Squibb, Celgene and Agois. T.Y., K.T., H.K., S.Y., M.S., H.S. M.O. and K.H. declare no competing interests. I.Y. has received grants from KAKENHI, AMED and Health, Labor and Welfare Policy Research Grants; and has received research funding from Nippon Medi-Physis; and has received honoraria from Chugai, Astra Zeneca and Nippon-Shinyaku. T.T. has received honoraria from Merck Sharp and Dohme, Takeda, Kyowa-Kirin, Bristol-Myers Squibb and Pfizer; and has received research funding from Chugai, Kyowa-Kirin, Sanofi, Astellas, TEIJIN PHARMA, Novartis, Fuji Pharma and Nippon Shinyaku; and served on an advisory board for Takeda and Novartis; and has participated in manuscript preparation as other financial interests for Janssen and Novartis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Goto, H., Kitawaki, T., Fujii, N. et al. Safety and efficacy of tisagenlecleucel in patients with relapsed or refractory B-cell lymphoma: the first real-world evidence in Japan. Int J Clin Oncol 28, 816–826 (2023). https://doi.org/10.1007/s10147-023-02334-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02334-w