Abstract
Background
A low-dose chemotherapy consisting of docetaxel, estramustine and dexamethasone was investigated for its beneficial effect and feasibility in Japanese patients with metastatic castration-resistant prostate cancer (CRPC).
Methods
Seventy-two Japanese patients with metastatic CRPC were enrolled to receive docetaxel (25 mg/m2 on days 2 and 9), estramustine phosphate (280 mg orally twice daily from day 1 to day 3 and from day 8 to day 10) and dexamethasone (0.5 mg orally twice daily) every 21 days.
Results
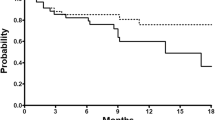
The median age of the patients was 72 years and 64 patients (89 %) had ≥grade 1 anemia at entry. The median total number of courses administered was 8.5 (range 1–93). Forty-two patients (58 %) had a prostate-specific antigen (PSA) decline of ≥50 %. The median progression-free survival and overall survival were 6 and 23 months, respectively. Fifteen patients (21 %) improved and 53 patients (74 %) were stable in their performance status. Of the 40 patients with bone pain, 25 patients (63 %) showed pain reduction. Among 71 patients assessable for their hemoglobin levels, 21 patients (30 %) achieved an increase of at least 1.0 g/dl. Of the 5 patients who terminated treatment because of ≥grade 3 toxicity, 4 patients had pneumonitis and one patient had anemia. Only one patient developed ≥grade 3 neutropenia.
Conclusions
The low-dose combination of docetaxel, estramustine and dexamethasone is active and tolerable with beneficial effects on serum PSA levels, performance status, anemia and bone pain in Japanese patients with CRPC. This regimen is a reasonable option for elderly patients with bone disease at risk of hematologic toxicity.




Similar content being viewed by others
References
Tannock IF, de Wit R, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512
Petrylak DP, Tangen CM, Hussain MH et al (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351:1513–1520
Shimazui T, Kawai K, Miyanaga N et al (2007) Three-weekly docetaxel with prednisone is feasible for Japanese patients with hormone-refractory prostate cancer: a retrospective comparative study with weekly docetaxel alone. Jpn J Clin Oncol 37:603–608
Naito S, Tsukamoto T, Koga H et al (2008) Docetaxel plus prednisolone for the treatment of metastatic hormone-refractory prostate cancer: a multicenter Phase II trial in Japan. Jpn J Clin Oncol 38:365–372
Ide H, Kikuchi E, Kono H et al (2010) Docetaxel in combination with prednisolone for hormone refractory prostate cancer. Jpn J Clin Oncol 40:79–84
Miura N, Numata K, Kusuhara Y et al (2010) Docetaxel-prednisolone combination therapy for Japanese patients with hormone-refractory prostate cancer: a single institution experience. Jpn J Clin Oncol 40:1092–1098
Nakagami Y, Ohori M, Sakamoto N et al (2010) Safety and efficacy of docetaxel, estramustine phosphate and hydrocortisone in hormone-refractory prostate cancer patients. Int J Urol 17:629–634
Berthold DR, Pond GR, Soban F et al (2008) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 26:242–245
Nishimura K, Nonomura N, Yasunaga Y et al (2000) Low doses of oral dexamethasone for hormone-refractory prostate carcinoma. Cancer 89:2570–2576
Hatano K, Nonomura N, Nishimura K et al (2011) Retrospective analysis of an oral combination of dexamethasone, uracil plus tegafur and cyclophosphamide for hormone-refractory prostate cancer. Jpn J Clin Oncol 41:253–259
Williams JF, Muenchen HJ, Kamradt JM et al (2000) Treatment of androgen-independent prostate cancer using antimicrotubule agents docetaxel and estramustine in combination: an experimental study. Prostate 44:275–278
Petrylak DP, Macarthur R, O’Connor J et al (1999) Phase I/II studies of docetaxel (Taxotere) combined with estramustine in men with hormone-refractory prostate cancer. Semin Oncol 26:28–33
Scher HI, Halabi S, Tannock I, Prostate Cancer Clinical Trials Working Group et al (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 26:1148–1159
Chowdhury S, Burbridge S, Harper PG (2007) Chemotherapy for the treatment of hormone-refractory prostate cancer. Int J Clin Pract 61:2064–2070
ten Tije AJ, Verweij J, Carducci MA et al (2005) Prospective evaluation of the pharmacokinetics and toxicity profile of docetaxel in the elderly. J Clin Oncol 23:1070–1077
Takenaka A, Yamada Y, Kurahashi T et al (2008) Combination chemotherapy with weekly docetaxel and estramustine for hormone refractory prostate cancer in Japanese patients. Int J Urol 15:106–109
Matsumoto A, Inoue A, Yokoi S et al (2009) Evaluation of docetaxel plus estramustine in the treatment of patients with hormone-refractory prostate cancer. Int J Urol 16:687–691
Leberbauer C, Boulme F, Unfried G et al (2005) Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood 105:85–94
Armstrong AJ, Garrett-Mayer ES, Yang YC et al (2007) A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res 13:6396–6403
Chao Y, Wu Q, Shepard C et al (2012) Hepatocyte induced re-expression of E-cadherin in breast and prostate cancer cells increases chemoresistance. Clin Exp Metastasis 29:39–50
Armstrong AJ, Garrett-Mayer E, Ou Yang YC et al (2007) Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J Clin Oncol 25:3965–3970
Petrylak DP, Ankerst DP, Jiang CS et al (2006) Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99–16. J Natl Cancer Inst 98:516–521
Saad F, Ruether D, Ernst S et al (2008) The Canadian Uro-Oncology Group multicentre phase II study of docetaxel administered every 3 weeks with prednisone in men with metastatic hormone-refractory prostate cancer progressing after mitoxantrone/prednisone. BJU Int 102:551–555
Berthold DR, Pond GR, de Wit R et al (2008) Survival and PSA response of patients in the TAX 327 study who crossed over to receive docetaxel after mitoxantrone or vice versa. Ann Oncol 19:1749–1753
Nishimura K, Nonomura N, Satoh E et al (2001) Potential mechanism for the effects of dexamethasone on growth of androgen-independent prostate cancer. J Natl Cancer Inst 93:1739–1746
Fizazi K, Le Maitre A, Hudes G et al (2007) Addition of estramustine to chemotherapy and survival of patients with castration-refractory prostate cancer: a meta-analysis of individual patient data. Lancet Oncol 8:994–1000
Eymard JC, Priou F, Zannetti A et al (2007) Randomized phase II study of docetaxel plus estramustine and single-agent docetaxel in patients with metastatic hormone-refractory prostate cancer. Ann Oncol 18:1064–1070
Caffo O, Sava T, Comploj E et al (2008) Docetaxel, with or without estramustine phosphate, as first-line chemotherapy for hormone-refractory prostate cancer: results of a multicentre, randomized phase II trial. BJU Int 102:1080–1085
Nelius T, Klatte T, Yap R et al (2006) A randomized study of docetaxel and dexamethasone with low- or high-dose estramustine for patients with advanced hormone-refractory prostate cancer. BJU Int 98:580–585
Machiels JP, Mazzeo F, Clausse M et al (2008) Prospective randomized study comparing docetaxel, estramustine, and prednisone with docetaxel and prednisone in metastatic hormone-refractory prostate cancer. J Clin Oncol 26:5261–5268
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
Conflict of interest
All authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hatano, K., Nishimura, K., Nakai, Y. et al. Weekly low-dose docetaxel combined with estramustine and dexamethasone for Japanese patients with metastatic castration-resistant prostate cancer. Int J Clin Oncol 18, 704–710 (2013). https://doi.org/10.1007/s10147-012-0429-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-012-0429-1