Abstract
Ingesting marine plastics is increasingly common in cetaceans, but little is known about their potential effects. Here, by utilizing 16S rRNA gene sequencing, we profiled the intestinal bacterial communities of a stranded Risso’s dolphin (Grampus griseus) which died because of the ingestion of rubber gloves. In this study, we explored the potential relationships between starvation raised by plastic ingestion with the dolphin gut microbiota. Our results showed significant differences in bacterial diversity and composition among the different anatomical areas along the intestinal tract, which may be related to the intestinal emptying process under starvation. In addition, the intestinal bacterial composition of the Risso’s dolphin showed both similarity and divergence to that of other toothed whales, suggesting potential roles of both host phylogeny and habitat sha** of the cetacean intestinal microbiome. Perhaps, the microbiota is reflecting a potentially disordered intestinal microbial profile caused by the ingestion of macro-plastics which led to starvation. Moreover, two operational taxonomic units (0.17% of the total reads) affiliated with Actinobacillus and Acinetobacter lwoffii were detected along the intestinal tract. These bacterial species may cause infections in immunocompromised dolphins which are malnourished. This preliminary study profiles the intestinal microbiota of a Risso’s dolphin, and provides an additional understanding of the potential relationships between starvation raised by ingesting macro-plastics with cetacean gut microbiota.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human-derived plastic debris in the ocean is ubiquitous and poses health risks to marine wildlife globally (Caruso et al. 2022; De Stephanis et al. 2013). Since cetaceans act as sentinel and indicator species for marine ecosystem assessment, there is no doubt that they are also threatened by marine plastic pollution. The effects of plastics on cetaceans are closely correlated with their species-specific diving and feeding behavior (Eisfeld-Pierantonio et al. 2022). For example, large filter-feeding baleen whales tend to intake microplastics (< 5 mm) when they engulf large amounts of water and mud, or from trophic transfer, while odontoceti predators are prone to ingest macro-plastics (25–1000 mm) when they capture plastic-shaped squids and fishes (Alava 2020; Alexiadou et al. 2019). According to the latest review, about 67.8% of cetacean species have been reported to interact with marine plastics, and the number of species has grown in the last decade (Eisfeld-Pierantonio et al. 2022). Specifically, compared to being entangled (34.4%; 31 species), a larger proportion of cetaceans was found to have ingested plastics (63.3%; 57 species), with macro-litter being the main issue for all. Even a tiny amount of ingested macro-plastic can have a huge impact on cetacean health, through blocking the gastrointestinal tract, followed by satiation, starvation, and malnutrition, and ultimately death can result. Even if the animal is able to survive a reduced quality of life, reproductive capacity can result (Eisfeld-Pierantonio et al. 2022; Gregory 1978, 1991). Moreover, the degradation of macro-plastics into micro-plastics can increase the ecotoxicological hazards, inducing the intestinal microbiota dysbiosis, in fish (Liao et al. 2022) and birds (Wang et al. 2021). The consequences of ingesting macro-plastics in cetaceans are becoming increasingly more serious as the ongoing COVID-19 pandemic has accelerated marine plastic pollution, like disposable face masks and gloves (Prata et al. 2020). Since the gut microbiota plays a critical role in host nutrition absorption, energy intake, and immune defense (Krajmalnik‐Brown et al. 2012; Takiishi et al. 2017), recent research has highlighted the possible adverse effects of starvation and malnutrition on the gut microbiota (Million et al. 2017; Vera-Ponce de León et al. 2021). The absence of the normal intestinal flora could trigger the susceptibility of a host to bacterial infections (Nell et al. 2010). Therefore, gut microbial dysbiosis may aid in our understanding on how ingesting macro-plastics influences cetacean fitness. However, up to now, little is known about the potential relationships between plastic ingestion with the cetacean gut microbiota.
The Risso’s dolphin (Grampus griseus), one of the largest toothed whales, is the sole species of the genus Grampus (family Delphinidae) and preys almost entirely on squids (Blanco et al. 2006). It has a cosmopolitan distribution, with a large range in water temperature (10–30 °C), water depth (under 10 m to deeper than 3800 m), and latitude (Jefferson et al. 2014; Kiszka et al. 2007). In China waters, the Risso’s dolphin is widely distributed from the South China Sea and Taiwan waters, to the East China Sea and the Yellow/Bohai Sea. It is currently listed as the national second-class protected animal. Even though a recent retrospective study indicated numerous stranding events (n = 62) of Risso’s dolphins in China waters from 1950 to 2018, there is still a lack of understanding about their cause of death and potential threats to their health (Liu et al. 2022). One example is the case of a female adult Risso’s dolphin which was stranded on the coast of the South China Sea near Zhanjiang City, Guangdong Province, in 2019 (Zeng et al. 2020). It was confirmed the dolphin ingested macro-plastics. However, as far as we know, the ingesting macro-plastics has not been described as a key issue for the conservation of the Risso’s dolphin, due to insufficient information on the potential physical and pathogenic effects of macro-plastics on cetaceans.
In this study, to follow up a previous report (Zeng et al. 2020), we profiled the intestinal bacterial communities of a Risso’s dolphin (Grampus griseus) stranded in South China Sea, and explored the potential relationships with starvation raised by macro-plastic ingestion.
Materials and methods
The Risso’s dolphin and intestinal content sampling
On July 10, 2019, a female Risso’s dolphin was stranded alive near Shimajiao waters in Xuwen County, Zhanjiang City, Guangdong Province, and died a half hour after being found (Zeng et al. 2020). The dolphin was then weighed, measured, and necropsied after death. As described by Zeng et al. (2020), the dolphin was emaciated, with extremely thin subcutaneous blubber; no apparent fatal traumas were detected, but two rubber gloves (> 30 cm) were found in the forestomach, and sands were detected in both the respiratory tract and esophagus. Moreover, the gastrointestinal tract was totally empty. It has been speculated that starvation and feebleness, following gastric blockage by rubber gloves, led to the live stranding and subsequent airway obstruction, which may have resulted in suffocation and finally death.
The intestinal tract was divided equally into three segments as the foregut, midgut, and hindgut. The luminal content from each intestinal segment was collected following the procedures outlined in a previous study (Wan et al. 2018). Three replicates from each intestinal segment were collected, resulting in a total of nine samples. All intestinal samples were stored in − 80 °C until DNA extraction.
DNA extraction, sequencing, reads processing, and statistical analysis
The metagenomic DNA from all the intestinal samples were extracted using the ZR fecal DNA kit (Zymo Research Incorporated, CA, USA), and then the concentration of extracted DNA was measured by a Nanodrop spectrophotometer. Qualified DNA products were amplified using the universal primers (338F: 5′-CCT AYG GGR BGC ASC AG-3′ and 806R: 5′-GGA CTA CNN GGG TAT CTA AT-3′), targeting the V3–V4 regions of the bacterial 16S rRNA gene. PCR products from each sample were then combined for library construction using TruSeq Nano DNA LT Library Prep Kit, and sequenced by the Miseq Illumina platform (Majorbio Company, Shanghai, China) (2 × 250 bp paired ends). The raw sequencing reads were preprocessed as described in Wan et al. (2021). In brief, after removing sequencing reads of poor quality and chimeras, the 16S rRNA gene sequences were clustered into operational taxonomic units (OTUs) with USEARCH (Edgar 2010) on the Galaxy platform at 97% nucleotide identity. Taxonomic affiliations were then assigned through the RDP classifier with a threshold of 0.7 (http://rdp.cme.msu.edu/). All sequences were randomly resampled to the minimum depth of 27,623 sequences per sample. Phylogenetic trees were constructed using FastTree tools (Price et al. 2009). The alpha-diversity indices were calculated using the Picante package in R, and compared between groups using the Wilcoxon rank sum test. Principal component analysis based on Bray–Curtis distances was further computed to evaluate the difference of microbial composition between groups. To identify biomarkers in each group, the linear discriminant analysis effect size (LEfSe, p < 0.05 and LDA score > 3.0) was analyzed online (http://huttenhower.sph.harvard.edu/galaxy/). To predict potential pathways from the 16S rRNA gene reads, the Functional Annotation of PROkaryotic TAXa (FAPROTAX) database was used (Louca et al. 2016).
Results and discussion
Overall microbial community structure and core microbiota
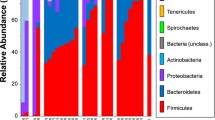
After rarefaction to 27,623 reads per sample, we obtained 62 OTUs in total, which is comparable with that from another stranded Risso’s dolphin (67 OTUs) found in Korea waters (Kim et al. 2019). The dominate phyla were Firmicutes (83.03%), followed by Fusobacteria (12.50%) and Bacteroidetes (4.28%), with five rare phyla accounting for the remaining 0.19% of the total sequencing reads (Fig. 1a). The five most abundant genera were Peptostreptococcus (40.60%), Paraclostridium (31.36%), Fusobacterium (11.76%), Vagococcus (5.94%), and Bacteroides (4.27%), which together were 93.93% in relative abundance (Fig. 1b). The top four abundant genera were also present in all intestinal samples, constituting of the abundant “core microbiota” of the Risso’s dolphin’s intestinal bacterial community. This is quite distinct from the microbial information of the Risso’s dolphin stranded in Korea waters, which was predominately Photobacterium (89.4%) (Kim et al. 2019). The Risso’s dolphin shared common gut microbial members associated with Firmicutes with a wide range of toothed whales, including the Chinese white dolphin (Sousa chinensis) (Wan et al. 2021), Yangtze finless porpoise (N. a. asiaeorientalis) (Wan et al. 2016), bottlenose dolphin (Tursiops truncatus) (Wan et al. 2022), melon-headed whales (Peponocephala electra) (Bai et al. 2022), short-finned pilot whales (Globicephala macrorhynchus) (Bai et al. 2021), pygmy (Kogia breviceps), and dwarf (K. sima) sperm whales (Erwin et al. 2017), whereas at the genus level, the Risso’s dolphin diverged from other toothed whales. For example, unlike Halomonas, Photobacterium, and Cetobacterium, detected as abundant taxa in the marine dolphins and porpoises (Wan et al. 2018, 2021, 2022), the Risso’s dolphin contained higher proportions of Vagococcus (5.94%) and Bacteroides (4.27%) (Fig. 1b). This may suggest that host phylogeny and habitat can help shape diverse gut microbial communities in different cetacean species, or that it may reflect the disordered intestinal microbial profile of the Risso’s dolphin which died of ingesting macro-plastics and starvation. Starvation may alter the morphology of intestinal epithelial cells and function of the gastrointestinal tract, and thus affect the microbiota colonized there (Okada et al. 2013). Therefore, physiological changes of the gastrointestinal tract during starvation may help explain how macro-plastic ingestion affect the microbial composition and diversity of the dolphin. However, the conclusion should be drawn with caution as only one individual was investigated in the present study.
Variations of bacterial diversity and composition in different intestinal regions
In general, the alpha diversity, estimated by five indices (Table 1), was not significantly different among the three intestinal regions (p > 0.05), which could be due to the limited sample size. However, Sobs (the number of observed OTUs), Chao 1, ACE, and Faith’s PD showed the highest values in the midgut, followed by the foregut and then the hindgut (Table 1). This is different with what was detected in a stranded Chinese white dolphin, which showed relatively higher diverse bacterial communities in the hindgut than the foregut and midgut (Wan et al. 2021). This difference between the Risso’s dolphin and the Chinese white dolphin may reflect differences in their intestinal emptying processes. Specifically, it is speculated that the Risso’s dolphin died not long after eating a prey item, after digesting her previous meal and emptying it to the midgut, leading to a higher midgut-associated bacterial diversity, whereas the Chinese white dolphin might have starved for a few days in the inland river, and emptied its chyme to the rectum. Therefore, the variation trend of the microbial diversity may change with host species or even with different nutritional statuses of the individuals. This may at least partly explain why there is currently no universal changing pattern of the intestinal microbial diversity of different cetacean species (Wan et al. 2018, 2021).
In agreement with previous findings (Wan et al. 2021), the bacterial community residing in the intestinal tract of the Risso’s dolphin exhibited stratifications among the foregut, midgut, and hindgut regions (Fig. 2). These three regions harbored distinct bacterial genera communities (Fig. 2a, b). Typically, Bacteroides and Porphyromonas, both belonging to Bacteroidota, were significantly enriched in the foregut, while Delftia (belonging to Proteobacteria) and Hathewaya (belonging to Firmicutes) were abundant in the midgut (Fig. 2c, d) (p < 0.05). The hindgut, the proportion of Rhizobiales (belonging to Proteobacteria), was statistically higher than that in the other two regions (Fig. 2c, d) (p < 0.05). This intestinal region-specificity of bacterial communities may be correlated with the different micro-environments present in different intestinal regions. For example, oxygen tension decreases greatly from the small to the large intestine, which help make the caecum and rectum of the large intestine favorable for enterohaemorrhagic Escherichia coli under anaerobic conditions (Woodward et al. 2019).
The differential analysis of bacterial composition among three intestinal regions. a Community heatmap analysis of nine samples at the genus level. The IDs on the right denote genera in Appendix Table 1. b Principal component analysis (PCA) of all samples based on Bray–Curtis distances. c The taxonomic cladogram visualized by LEfSe analysis. Red, blue, and green areas represent foregut-, midgut-, and hindgut-enriched bacterial taxa, respectively. d Linear discriminant analysis (LDA) scores (log10) obtained from the LEfSe analysis showed the biomarker bacteria taxa (LDA score > 3 with p < 0.05). The IDs on the left denote the genus information corresponding to the panel c
Functional potentials
Instead of exploring the general functional pathways of microbial communities along the intestinal tract, here we mainly focused on the potential pathogens, as well as plastic degradation-associated functions. In total, only 2 OTUs (0.17% of the total reads) affiliated with Actinobacillus (identity 99.53%) and Acinetobacter lwoffii (identity 100%), respectively, were potentially pathogenic by FAPROTAX analysis. The pathogenicity of these organisms has been experimentally verified in the literature. Specifically, Actinobacillus species (belonging to Pasteurellales) can cause actinomycosis, potent septicaemia, and fatal pneumonia in mammals (Rycroft and Garside 2000). And A. lwoffii (belonging to Pseudomonadales) can cause chronic gastritis (Rathinavelu et al. 2003). It’ is noticeable that the extremely low abundance of potential pathogens may be due to the low biomass of intestinal bacteria in the starving dolphin. Interestingly, in the present study, two rare OTUs, belonging to Delftia tsuruhatensis and A. lwoffii, respectively, were detected, both of which are related to plastic-degradation (Liang et al. 2008; Shigematsu et al. 2003). These bacterial species may be involved in the utilization of microplastics in the dolphin’s gut, but further research is necessary to verify this possibility.
In conclusion, this study profiled the bacterial community composition and predicted functions along different intestinal regions of an adult Risso’s dolphin which died of ingesting rubber gloves, leading to starvation and malnutrition. Even though more stranding cases are necessary to obtain reliable results, this report provides the first microbial identification in the different intestinal regions of the Risso’s dolphin, which is associated with the potential relationships with starvation raised by ingesting macro-plastics. This result could serve as a reference for exploring the interactions between gut microbial dysbiosis and ingesting macro-plastics. This study highlights the dangers of the ingestion of plastics by cetaceans, especially since there is an increase in the disposable of plastics due to the COVID-19 pandemic.
Data availability
All raw sequencing reads have been deposited in the NCBI database with accession number PRJNA876954.
References
Alava JJ (2020) Modeling the bioaccumulation and biomagnification potential of microplastics in a cetacean foodweb of the northeastern pacific: a prospective tool to assess the risk exposure to plastic particles. Front Mar Sci 7:566101. https://doi.org/10.3389/fmars.2020.566101
Alexiadou P, Foskolos I, Frantzis A (2019) Ingestion of macroplastics by odontocetes of the Greek Seas, Eastern Mediterranean: Often deadly! Mar Pollut Bull 146:67–75. https://doi.org/10.1016/j.marpolbul.2019.05.055
Bai S, Zhang P, Lin M, Lin W, Yang Z, Li S (2021) Microbial diversity and structure in the gastrointestinal tracts of two stranded short-finned pilot whales (Globicephala macrorhynchus) and a pygmy sperm whale (Kogia breviceps). Integr Zool 16(3):324–335. https://doi.org/10.1111/1749-4877.12502
Bai S, Zhang P, Zhang X, Yang Z, Li S (2022) Gut microbial characterization of melon-headed whales (Peponocephala electra) stranded in China. Microorganisms 10(3):572. https://doi.org/10.3390/microorganisms10030572
Blanco C, Raduán MÁ, Raga JA (2006) Diet of Risso’s dolphin (Grampus griseus) in the western Mediterranean Sea. Sci Mar 70(3):407–411. https://doi.org/10.3989/scimar.2006.70n3407
Caruso G, Bergami E, Singh N, Corsi I (2022) Plastic occurrence, sources, and impacts in Antarctic environment and biota. Water Biol Security 1(2):100034. https://doi.org/10.1016/j.watbs.2022.100034
De Stephanis R, Giménez J, Carpinelli E, Gutierrez-Exposito C, Cañadas A (2013) As main meal for sperm whales: plastics debris. Mar Pollut Bull 69(1–2):206–214. https://doi.org/10.1016/j.marpolbul.2013.01.033
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26(19):2460–2461. https://doi.org/10.1093/bioinformatics/btq461
Eisfeld-Pierantonio SM, Pierantonio N, Simmonds MP (2022) The impact of marine debris on cetaceans with consideration of plastics generated by the COVID-19 pandemic. Environ Pollut 300:118967. https://doi.org/10.1016/j.envpol.2022.118967
Erwin PM, Rhodes RG, Kiser KB, Keenan-Bateman TF, McLellan WA, Pabst DA (2017) High diversity and unique composition of gut microbiomes in pygmy (Kogia breviceps) and dwarf (K. sima) sperm whales. Sci Rep 7(1):7205. https://doi.org/10.1038/s41598-017-07425-z
Gregory MR (1978) Accumulation and distribution of virgin plastic granules on New Zealand beaches. New Zeal J Mar Freshwater Res 12(4):399–414. https://doi.org/10.1080/00288330.1978.9515768
Gregory MR (1991) The hazards of persistent marine pollution: drift plastics and conservation islands. J Roy Soc New Zeal 21(2):83–100. https://doi.org/10.1080/03036758.1991.10431398
Jefferson TA, Weir CR, Anderson RC, Ballance LT, Kenney RD, Kiszka JJ (2014) Global distribution of Risso’s dolphin Grampus griseus: a review and critical evaluation. Mammal Rev 44(1):56–68. https://doi.org/10.1111/mam.12008
Kim SW, Han SJ, Lee YR, Kim BY, Park SC (2019) First report of a Risso’s dolphin (Grampus griseus) stranded in Jeju Island, Republic of Korea: findings from necropsy, histopathology and microbiome analysis. Vet Rec Case Rep 7(4):e000860. https://doi.org/10.1136/vetreccr-2019-000860
Kiszka J, Macleod K, Van Canneyt O, Walker D, Ridoux V (2007) Distribution, encounter rates, and habitat characteristics of toothed cetaceans in the Bay of Biscay and adjacent waters from platform-of-opportunity data. Ices J Marine Sci 64(5):1033–1043. https://doi.org/10.1093/icesjms/fsm067
Krajmalnik-Brown R, Ilhan ZE, Kang DW, DiBaise JK (2012) Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract 27(2):201–214. https://doi.org/10.1177/0884533611436116
Liang D, Zhang T, Fang H, He J (2008) Phthalates biodegradation in the environment. Appl Microbiol Biotechnol 80(2):183–198. https://doi.org/10.1007/s00253-008-1548-5
Liao H, Liu S, Junaid M, Gao D, Ai W, Chen G, Wang J (2022) Di-(2-ethylhexyl) phthalate exacerbated the toxicity of polystyrene nanoplastics through histological damage and intestinal microbiota dysbiosis in freshwater Micropterus salmoides. Water Res 219:118608. https://doi.org/10.1016/j.watres.2022.118608
Liu M, Lin M, Li S (2022) Species diversity and spatiotemporal patterns based on cetacean stranding records in China, 1950–2018. Sci Total Environ 822:153651. https://doi.org/10.1016/j.scitotenv.2022.153651
Louca S, Parfrey LW, Doebeli M (2016) Decoupling function and taxonomy in the global ocean microbiome. Science 353(6305):1272–1277. https://doi.org/10.1126/science.aaf4507
Million M, Diallo A, Raoult D (2017) Gut microbiota and malnutrition. Microb. Pathogenesis 106:127–138. https://doi.org/10.1016/j.micpath.2016.02.003
Nell S, Suerbaum S, Josenhans C (2010) The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol 8(8):564–577. https://doi.org/10.1038/nrmicro2403
Okada T, Fukuda S, Hase K, Nishiumi S, Izumi Y, Yoshida M, Hagiwara T, Kawashima R, Yamazaki M, Oshio T, Otsubo T, Inagaki-Ohara K, Kakimoto K, Higuchi K, Kawamura YI, Ohno H, Dohi T (2013) Microbiota-derived lactate accelerates colon epithelial cell turnover in starvation-refed mice. Nat Commun 4(1):1654. https://doi.org/10.1038/ncomms2668
Prata JC, Silva AL, Walker TR, Duarte AC, Rocha-Santos T (2020) COVID-19 pandemic repercussions on the use and management of plastics. Environ Sci Technol 54(13):7760–7765. https://doi.org/10.1021/acs.est.0c02178
Price MN, Dehal PS, Arkin AP (2009) FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol 26(7):1641–1650. https://doi.org/10.1093/molbev/msp077
Rathinavelu S, Zavros Y, Merchant JL (2003) Acinetobacter lwoffii infection and gastritis. Microbes Infect 5(7):651–657. https://doi.org/10.1016/S1286-4579(03)00099-6
Rycroft AN, Garside LH (2000) Actinobacillus species and their role in animal disease. Vet J 159(1):18–36. https://doi.org/10.1053/tvjl.1999.0403
Shigematsu T, Yumihara K, Ueda Y, Numaguchi M, Morimura S, Kida K (2003) Delftia tsuruhatensis sp. nov., a terephthalate-assimilating bacterium isolated from activated sludge. Int J Syst Evol Microbiol 53(5):1479–1483. https://doi.org/10.1099/ijs.0.02285-0
Takiishi T, Fenero CIM, Câmara NOS (2017) Intestinal barrier and gut microbiota: sha** our immune responses throughout life. Tissue Barriers 5(4):e1373208. https://doi.org/10.1080/21688370.2017.1373208
Vera-Ponce de León A, Jahnes BC, Otero-Bravo A, Sabree ZL (2021) Microbiota perturbation or elimination can inhibit normal development and elicit a starvation-like response in an omnivorous model invertebrate. mSystems 6(4):802–21. https://doi.org/10.1128/mSystems.00802-21
Wan X, Li J, Cheng Z, Ao M, Tian R, Mclaughlin RW, Zheng J, Wang D (2021) The intestinal microbiome of an Indo-Pacific humpback dolphin (Sousa chinensis) stranded near the Pearl River Estuary. China Integr Zool 16(3):287–299. https://doi.org/10.1111/1749-4877.12477
Wan X, Li J, Tian R, McLaughlin R, Hao Y, Wu J, Wang Z, Fan F, Wang D, Zheng J (2022) The effects of human care on the blowhole and gut microbiotas of two cohabiting dolphin species based on a year-round surveillance. Front Mar Sci 9:2224. https://doi.org/10.3389/fmars.2022.1024117
Wan X, McLaughlin RW, Zheng J, Hao Y, Fan F, Tian R, Wang D (2018) Microbial communities in different regions of the gastrointestinal tract in East Asian finless porpoises (Neophocaena asiaeorientalis sunameri). Sci Rep 8(1):1–10. https://doi.org/10.1038/s41598-018-32512-0
Wan X, Ruan R, McLaughlin RW, Hao Y, Zheng J, Wang D (2016) Fecal bacterial composition of the endangered Yangtze finless porpoises living under captive and semi-natural conditions. Curr Microbiol 72(3):306–314. https://doi.org/10.1007/s00284-015-0954-z
Wang L, Nabi G, Yin L, Wang Y, Li S, Hao Z, Li D (2021) Birds and plastic pollution: recent advances. Avian Res 12:1–9. https://doi.org/10.1186/s40657-021-00293-2
Woodward SE, Krekhno Z, Finlay BB (2019) Here, there, and everywhere: how pathogenic Escherichia coli sense and respond to gastrointestinal biogeography. Cell Microbiol 21(11):e13107. https://doi.org/10.1111/cmi.13107
Zeng Q, Aierken R, Li J, Zhong M, Zhu Q, Zheng J (2020) Pathological anatomy and death cause of a stranded Risso’s dolphin (Grampus griseus). Acta Theriologica Sinica 40(2):152–161. https://doi.org/10.16829/j.slxb.150364. (in Chinese with English Abstract)
Acknowledgements
The authors would like to thank the staff from the Bureau of Agriculture and Rural Affairs of Xuwen City, for their endeavor of rescuing the live-stranded Risso’s dolphin reported in this study. We also thank Mr. Renchun Dong, Shenwei Tong, and Dr. Qianhui Zeng for their help in the postmortem examination and sample collecting.
Funding
This work was partly supported by grants from China Postdoctoral Science Foundation (No. 2020M682530), the National Natural Science Foundation of China (No. 31870372), the Bureau of Science & Technology for Development, and the Chinese Academy of Sciences (No. ZSSD-004).
Author information
Authors and Affiliations
Contributions
**aoling Wan: methodology, software, funding acquisition, writing—original draft preparation. Jia Li: investigation, data curation. Mengxue Ao: methodology, formal analysis. Richard William McLaughlin: formal analysis, writing—review and editing. Fei Fan: writing—review and editing. Ding Wang: writing—review and editing. **song Zheng: conceptualization, supervision, funding acquisition, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
Carcass processing and sampling permission in this study had been authorized by the Bureau of Agriculture and Rural Affairs of Xuwen City, and were conducted in accordance with the Regulations of the People’s Republic of China for the Implementation of Wild Aquatic Animal Protection (promulgated in 1993), adhering to all ethical guidelines and legal requirements in China.
Consent for publication
All authors consent to the publication of this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wan, X., Li, J., Ao, M. et al. The intestinal microbiota of a Risso’s dolphin (Grampus griseus): possible relationships with starvation raised by macro-plastic ingestion. Int Microbiol 26, 1001–1007 (2023). https://doi.org/10.1007/s10123-023-00355-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-023-00355-z