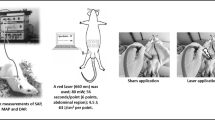
Abstract
A previous work indicates that the red LASER (660 nm) induces vascular relaxation by nitric oxide (NO)–dependent mechanism. NO activates soluble guanylate cyclase (sGC) which produces cGMP, the main effector in the vasodilation pathway. An interesting pharmacological strategy is to control the levels of intracellular cGMP, preventing its efflux (with multidrug-resistant protein blockers, such as MK-571), or preventing its degradation (such as sildenafil, which inhibits the enzyme responsible for cGMP degradation, the phosphodiesterase-5 PDE5). This study aimed to look for pharmacological strategies to improve vasodilation LASER effect in normotensive and hypertensive rats (l-NAME model). The vascular reactivity study was performed in isolated aortic rings from normotensive and hypertensive rats, with a single LASER application and sodium nitroprusside (SNP) treatment. In aortic rings from normotensive rats, MK-571 and sildenafil potentiated the relaxation induced by LASER, compared to control. The vasodilation induced by SNP was potentiated by MK-571 and sildenafil, compared to control. In aortic rings from hypertensive rats, vasodilation effect induced by LASER and by SNP was potentiated just by MK-571, compared to control, with no potentiation by sildenafil. In addition, it was seen that the withdrawal of nitric oxide stocks carried out by l-cysteine is capable of being reversed with the use of the SNP. The results support the evidence that the vasodilation induced by red LASER is potentiated by MK-571 and sildenafil in aortic rings from normotensive rats. However, in aortic rings from l-NAME hypertensive rats, the potentiation in vasodilation was induced just by MK-571.









Similar content being viewed by others
References
Jung O, Gechter JL, Wunder C et al (2013) Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens 31:766–774
Mozaffarian D, Benjamin EJ, Go AS et al (2015) Heart disease and stroke statistics-2015: update a report from the American Heart Association. Circulation 131(4):e29-322
Dodhia H, Phillips K, Zannou MI et al (2012) Modelling the impact on avoidable cardiovascular disease burden and costs of interventions to lower SBP in the England population. J Hypertens 30(1):217–226
Krousel-Wood M (2015) Hypertension and health behaviors in females across the lifespan. Am J Med Sci 350(1):36–41
Haynes RB, McDonald HP, Garg A (2002) Hel** patients follow prescribed treatment: clinical applications. JAMA 288(22):2880–2883
Krousel-Wood MA, Muntner P, Islam T et al (2009) Barriers to and determinants of medication adherence in hypertension management: perspective of the cohort study of medication adherence among older adults. Med Clin North Am 93(3):753–769
Byun JW, Babithe S, Kim EK et al (2015) A successful helium-neon LASER and topical tacrolimus combination therapy in one child with vitiligo. Dermatol Ther 28(6):333–335
Erceg A, de Jong EMJG, van de Kerkhof PCM, Seyger MMB (2013) The efficacy of pulsed dye LASER treatment for inflammatory skin diseases: a systematic review. J Am Acad Dermatol 69(4):609–615.e8
Tomimura S, Silva BPA, Sanches IC et al (2014) Hemodynamic effect of LASER therapy in spontaneously hypertensive rats. Arq Bras Cardiol 103(2):161–164
Furchgott RF et al (1955) Relaxation of arterial strips by light, and the influence of drugs on this photodynamic effect. J Pharrnacol and Exp Therap 113:29
Furchgott RF, Ehrreich SJ (1961) Greenblatt E (1955) The photoactivated relaxation of smooth muscle of rabbit aorta. J Gen Physiol 44:499–519
Matsunaga K, Furchgott RF (1989) Interactions of light and sodium nitrite in producing relaxation of rabbit aorta. J Pharmacol Exp Ther 248(2):687–695
Plass CA, Loew HG, Podesser BK et al (2012) Light-induced vasodilation of coronary arteries and its possible clinical implication. Ann Thorac Surg 93(4):1181–1186
Oishi JC, de Moraes TF, Buzinari TC et al (2017) Hypotensive acute effect of photobiomodulation therapy on hypertensive rats. Life Sci 1(178):56–60
de Moraes LHO, Mancini MW, Almeida-Lopes L, Rodrigues GJ (2021) Violet LED induces vasodilation in rat aortic rings by soluble guanylate cyclase-dependent mechanism and increases SOD activity. Lasers Med Sci. https://doi.org/10.1007/s10103-021-03293-2
Bagheri HS, Mousavi M, Rezabakhsh A et al (2018) Low-level laser irradiation at a high power intensity increased human endothelial cell exosome secretion via Wnt signaling. Lasers Med Sci 33:1131–1145. https://doi.org/10.1007/s10103-018-2495-8
Rapoport RM, Draznin MB, Murad F (1983) Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature 306:174–176
Gewaltig MT, Kojda G (2002) Vasoprotection by nitric oxide: mechanism and therapeutic potential. Cardiovsc Res 55(2):250–260
Jedlitschky G, Burchell B, Keppler D (2000) The multidrug resistence protein 5 function as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem 275(39):30069–74
Sassi Y, Lipkaia L, Vandecasteele G et al (2008) Multidrug resistance-associated protein 4 regulates cAMP-dependentsignaling pathways and controls human and rat SMC proliferation. J Clin Invest 118:2747–2757
Hara Y, Sassi Y, Guibert C et al (2011) Inhibition of MRP4 prevents and reverses pulmonary hypertension in mice. J Clin Invest 121(7):2888–2897
Mattiello T, Guerriero R, Lotti LV et al (2011) Aspirin extrusion from human platelets through multidrug resistanceprotein-4-mediated transport: evidence of a reduced drug action in patientsafter coronary artery bypass grafting. J Am Coll Cardio 58(7):752–761
Rodrigues GJ, Lunardi CN, Lima RG, Santos CX, Laurindo FR, da Silva RS, Bendhack LM (2008) Vitamin C improves the effect of a new nitric oxide donor on the vascular smooth muscle from renal hypertensive rats. Nitric Oxide 18(3):176–183. https://doi.org/10.1016/j.niox.2007.12.002
Paulis L, Pechanova O, Zicha J, Barta A, Gardlik R, Celec P, Kunes J, Simko F (2010) Melatonin interactions with blood pressure and vascular function during L-NAMEinduced hypertension. J Pineal Res 48(2):102–108. https://doi.org/10.1111/j.1600-079X.2009.00732.x
Alencar JL, Lobysheva I, Geffard M, Sarr M, Schott C, Schini-Kerth VB, Nepveu F, Stoclet JC, Muller B (2003) Role of S-nitrosation of cysteine residues in long-lasting inhibitory effect of nitric oxide on arterial tone. Mol Pharmacol 63(5):1148–1158. https://doi.org/10.1124/mol.63.5.1148
Jedlitschky G, Burchell B, Keppler D (2000) The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem 275(39):30069–30074. https://doi.org/10.1074/jbc.M005463200
Hara Y, Sassi Y, Guibert C, Gambaryan N, Dorfmüller P, Eddahibi S, Lompré AM, Humbert M, Hulot JS (2011) Inhibition of MRP4 prevents and reverses pulmonary hypertension in mice. J Clin Invest. 121(7):2888–97. https://doi.org/10.1172/JCI45023
Decouture B, Dreano E, Belleville-Rolland T, Kuci O, Dizier B, Bazaa A, Coqueran B, Lompre AM, Denis CV, Hulot JS, Bachelot-Loza C, Gaussem P (2015) Impaired platelet activation and cAMP homeostasis in MRP4-deficient mice. Blood 126(15):1823–30. https://doi.org/10.1182/blood-2015-02-631044
Cornwell TL, Pryzwansky KB, Wyatt TA, Lincoln TM (1991) Regulation of sarcoplasmic reticulum protein phosphorylation by localized cyclic GMP-dependent protein kinase in vascular smooth muscle cells. Mol Pharmacol 40(6):923–931
Furukawa K, Ohshima N, Tawada-Iwata Y, Shigekawa M (1991) Cyclic GMP stimulates Na+/Ca2+ exchange in vascular smooth muscle cells in primary culture. J Biol Chem 266(19):12337–12341
Fisher SK, Novak JE, Agranoff BW (2002) Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem 82(4):736–754. https://doi.org/10.1046/j.1471-4159.2002.01041.x
Lincoln TM, Komalavilas P, Cornwell TL (1994) Pleiotropic regulation of vascular smooth muscle tone by cyclic GMP-dependent protein kinase. Hypertension 23(6 Pt 2):1141–1147. https://doi.org/10.1161/01.hyp.23.6.1141
Martinelli AM, Rodrigues CNDS, Moraes TF, Rodrigues GJ (2018) In Endothelial cells, the activation or stimulation of soluble guanylyl cyclase induces the nitric oxide production by a mechanism dependent of nitric oxide synthase activation. J Pharm Pharm Sci 21(1):38–45. https://doi.org/10.18433/jpps29578
Tare M, Parkington HC, Coleman HA, Neild TO, Dusting GJ (1990) Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature 346(6279):69–71. https://doi.org/10.1038/346069a0
Wanstall JC, Jeffery TK, Gambino A, Lovren F, Triggle CR (2001) Vascular smooth muscle relaxation mediated by nitric oxide donors: a comparison with acetylcholine, nitric oxide and nitroxyl ion. Br J Pharmacol 134(3):463–472. https://doi.org/10.1038/sj.bjp.0704269
Hebeda CB, Teixeira SA, Tamura EK, Muscará MN, de Mello SB, Markus RP, Farsky SH (2011) Nitric oxide modulates lipopolysaccharide-induced endothelial platelet endothelial cell adhesion molecule expression via interleukin-10. Clin Exp Immunol 165(2):172–9. https://doi.org/10.1111/j.1365-2249.2011.04396.x
Keszler A, Lindemer B, Hogg N, Lohr NL (2019) Ascorbate attenuates red light mediated vasodilation: potential role of S-nitrosothiols. Redox Biol 20:13–18. https://doi.org/10.1016/j.redox.2018.09.008
Choi H, Tostes RC, Webb RC (2011) S-Nitrosylation inhibits protein kinase C-mediated contraction in mouse aorta. J Cardiovasc Pharmacol 57(1):65–71. https://doi.org/10.1097/FJC.0b013e3181fef9cb
Kumar M et al (2010) Efficacy of piperine, an alkaloidal constituent of pepper on nitric oxide, antioxidants and lipid peroxidation markers in L-NAME induced hypertensive rats. Int J Res Pharm Sci 1:300–307
Moncada S, Rees DD, Schulz R, Palmer RM (1991) Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc Natl Acad Sci U S A 88(6):2166–2170. https://doi.org/10.1073/pnas.88.6.2166
Mullershausen F, Russwurm M, Thompson WJ, Liu L, Koesling D, Friebe A (2001) Rapid nitric oxide-induced desensitization of the cGMP response is caused by increased activity of phosphodiesterase type 5 paralleled by phosphorylation of the enzyme. J Cell Biol 155(2):271–8. https://doi.org/10.1083/jcb.200107001
Brandes RP, Kim D, Schmitz-Winnenthal FH, Amidi M, Gödecke A, Mülsch A, Busse R (2000) Increased nitrovasodilator sensitivity in endothelial nitric oxide synthase 11 knockout mice: role of soluble guanylyl cyclase. Hypertension 35(1 Pt 2):231–236. https://doi.org/10.1161/01.hyp.35.1.231
Acknowledgements
Special thanks to DMC Equipment for lending the equipment generously to carry out the experiments.
Funding
This study was financed in part by the São Paulo Research Foundation (FAPESP 2018/10588-9) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) – (Finance Code 001).
Author information
Authors and Affiliations
Contributions
LM and GR: Conceptualization; LM and GR: data curation; LM, BT, NM, and GR: formal analysis; GR: funding acquisition; LM: investigation; GR: methodology; GR: project administration; LM: resources; GR: supervision; GR: validation; GR: visualization; LM: roles/writing—original draft; LM and BT: writing—review and editing.
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Moraes, L.H.O., Terroni, B., da Silva Mayer, N.F. et al. Multidrug-resistant protein inhibitor and phosphodiesterase inhibitor potentiate the vasodilator effect induced by photobiomodulation in isolated aortic rings. Lasers Med Sci 37, 1209–1216 (2022). https://doi.org/10.1007/s10103-021-03374-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03374-2