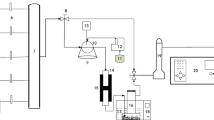
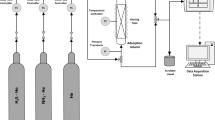
Abstract
Air pollution caused by sulphur dioxide (SO2) emissions is pervasive and has severe health and environmental consequences. In recent years, global SO2 emissions have decreased dramatically due to the widespread adoption of cleaner technologies and stricter emission regulations. The availabilities of highly concentrated SO2 separation prompted this study’s focus on reducing SO2 emissions, particularly for low-concentrated SO2 (100 ppm), employing ferric oxide nanoparticles. Upon thermal degradation of the adsorptive ferric oxide, both functional Fe–O and ferric oxide were observed. A 3:1 mixture of sodium hydroxide and ethanol was used to chemically activate the surface of the adsorbent, increasing both the pH and surface area from 6.5 to 10 and 183 to 531 m2 g−1, respectively. Maximum adsorption capacity (477.5 mg g−1) and maximum adsorption percentage (97.89%) were highest for the lowest SO2 concentration (56 mg m−3) compared to the other two SO2 concentrations (65 and 73 mg m−3). The Freundlich isotherm outperforms the other four models with the highest correlation coefficient (R2 = 0.9897). SO2 adsorption was physisorption, as the thermodynamic values, change of enthalpy and free energy decreased from −902 to −2052 kJ mol−1 and −243 to −562 kJ mol−1 as the temperature of the adsorption process ascended from room temperature to 70 °C. The SO2 was successfully removed from the adsorbent using temperature-programmed desorption analysis, with a maximal discharge of 1681 mg m−3 of SO2 and enhanced ferric oxide. Effective SO2 adsorption by the adsorbent for up to four cycles suggests that SO2 capture costs could be decreased.
Graphical abstract

A Sustainable Approach: Reducing Sulphur Dioxide Emissions via Adsorption on Chemically Altered Iron Oxide Nanoparticles









































Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
References
Akl AA, Mahmoud SA, AL-Shomar SM, Hassanien AS (2018) Improving microstructural properties and minimizing crystal imperfections of nanocrystalline Cu2O thin films of different solution molarities for solar cell applications. Mater Sci Semicond Process 74:183–192. https://doi.org/10.1016/j.mssp.2017.10.007
An L, Jia X, Liu Y (2019) Adsorption of SO2 molecules on Fe-doped carbon nanotubes: the first principles study. Adsorption 25:217–224. https://doi.org/10.1007/s10450-019-00026-4
Auvinen P, Kinnunen NM, Hirvi JT et al (2021) Effects of NO and NO2 on fresh and SO2 poisoned methane oxidation catalyst—Harmful or beneficial? Chem Eng J 417:128050. https://doi.org/10.1016/j.cej.2020.128050
Bahrin D, Subagjo S, Susanto H (2016) Kinetic study on the SO2 adsorption using CuO/γ-Al2O3 adsorbent. Bull Chem React Eng Catal 11:93. https://doi.org/10.9767/bcrec.11.1.425.93-100
Barpaga D, LeVan MD (2016) Functionalization of carbon silica composites with active metal sites for NH3 and SO2 adsorption. Microporous Mesoporous Mater 221:197–203. https://doi.org/10.1016/j.micromeso.2015.09.044
Burns J, Boogaard H, Polus S et al (2020) Interventions to reduce ambient air pollution and their effects on health: an abridged Cochrane systematic review. Environ Int 135:105400. https://doi.org/10.1016/j.envint.2019.105400
Cai M, Liu X, Zhu T et al (2020) Simultaneous removal of SO2 and NO using a spray dryer absorption (SDA) method combined with O3 oxidation for sintering/pelleting flue gas. J Environ Sci 96:64–71. https://doi.org/10.1016/j.jes.2020.04.018
Chen F, Lai D, Guo L et al (2021) Deep desulfurization with record SO2 adsorption on the metal–organic frameworks. J Am Chem Soc 143:9040–9047. https://doi.org/10.1021/jacs.1c02176
Cofala J, Amann M, Gyarfas F et al (2004) Cost-effective control of SO2 emissions in Asia. J Environ Manage 72:149–161. https://doi.org/10.1016/j.jenvman.2004.04.009
Cortés-Arriagada D, Villegas-Escobar N, Ortega DE (2018) Fe-doped graphene nanosheet as an adsorption platform of harmful gas molecules (CO, CO2, SO2 and H2S), and the co-adsorption in O2 environments. Appl Surf Sci 427:227–236. https://doi.org/10.1016/j.apsusc.2017.08.216
Cui H, Zhang X, Chen D (2018) Borophene: a promising adsorbent material with strong ability and capacity for SO2 adsorption. Appl Phys A 124:1–8. https://doi.org/10.1007/s00339-018-2064-9
Davini P (1996) Investigation of the SO2 adsorption properties of Ca(OH)2-fly ash systems. Fuel 75:713–716. https://doi.org/10.1016/0016-2361(95)00303-7
Davini P (2002) Influence of surface properties and iron addition on the SO2 adsorption capacity of activated carbons. Carbon 40:729–734. https://doi.org/10.1016/s0008-6223(01)00161-0
Deng Z-Y, Zhang J-M, Xu K-W (2015) First-principles study of SO2 molecule adsorption on the pristine and Mn-doped boron nitride nanotubes. Appl Surf Sci 347:485–490. https://doi.org/10.1016/j.apsusc.2015.04.116
Fan YL, Zhang HP, Yin MJ et al (2020) High adsorption capacity and selectivity of SO2 over CO2 in a metal–organic framework. Inorg Chem 60:4–8. https://doi.org/10.1021/acs.inorgchem.0c02893
Feng T, Zhang S, Li J et al (2020) Experimental and thermodynamic study on SO2 reduction to elemental sulfur by activated coke and pyrolysis gas: Influence of the reaction atmosphere. Int J Hydrogen Energy 45:20120–20131. https://doi.org/10.1016/j.ijhydene.2020.05.087
Fu D, Guo W, Li M et al (2014) Adsorption and reaction mechanisms of SO2 on Rh(111) surface: a first-principle study. J Mol Struct 1062:68–76. https://doi.org/10.1016/j.molstruc.2014.01.035
Gao Z, Liu X, Li A et al (2019) Adsorption behavior of mercuric oxide clusters on activated carbon and the effect of SO2 on this adsorption: a theoretical investigation. J Mol Model 25:1. https://doi.org/10.1007/s00894-019-4026-3
Guo Y, Li Y, Zhu T, Ye M (2012) Effects of concentration and adsorption product on the adsorption of SO2 and NO on activated carbon. Energy Fuels 27:360–366. https://doi.org/10.1021/ef3016975
Hamzehlouyan T, Sampara CS, Li J et al (2016) Kinetic study of adsorption and desorption of SO2 over γ-Al2O3 and Pt/γ-Al2O3. Appl Catal B 181:587–598. https://doi.org/10.1016/j.apcatb.2015.08.003
Han X, Yang S, Schröder M (2019) Porous metal–organic frameworks as emerging sorbents for clean air. Nat Rev Chem 3:108–118. https://doi.org/10.1038/s41570-019-0073-7
Hao R, Luo Y, Qian Z et al (2020) Simultaneous removal of SO2, NO and HgO using an enhanced gas phase UV-AOP method. Sci Total Environ 734:139266. https://doi.org/10.1016/j.scitotenv.2020.139266
Hosseiniebalam F, Ghaffarpasand O (2015) The effects of emission sources and meteorological factors on sulphur dioxide concentration of great Isfahan, Iran. Atmos Environ 100:94–101. https://doi.org/10.1016/j.atmosenv.2014.10.012
Hungerford J, Bhattacharyya S, Tumuluri U et al (2018) DMOF-1 as a representative MOF for SO2 adsorption in both humid and dry conditions. J Phys Chem C 122:23493–23500. https://doi.org/10.1021/acs.jpcc.8b06819
Jędrusik J, Kalinowski E, Jędrusik M (1998) A method of reducing the SO2 emission from power boilers. Chem Prot Environ 3:79–85. https://doi.org/10.1007/978-1-4757-9664-3_9
Jeyabharathi D, Thava AM, Idas SJP, Sangeetha T (2021) Waste management in smart cities using blockchaining technology. In: Blockchain for smart cities. pp 171–181. https://doi.org/10.1016/b978-0-12-824446-3.00014-4
Jeyapaul AS, Ganesapillai M (2020) Dual packed bed adsorption of sulphur dioxide from surface modified haematite/III-ferric oxide: characterization of the mass transfer zone. S Afr J Chem Eng 33:95–102. https://doi.org/10.1016/j.sajce.2020.06.008
Kang DW, Ju SE, Kim DW et al (2020) Emerging porous materials and their composites for NH3 gas removal. Adv Sci 7:2002142. https://doi.org/10.1002/advs.202002142
Kharol SK, Fioletov V, McLinden CA et al (2020) Ceramic industry at Morbi as a large source of SO2 emissions in India. Atmos Environ 223:117243. https://doi.org/10.1016/j.atmosenv.2019.117243
Kumar N, Mukherjee S, Harvey-Reid NC et al (2021) Breaking the trade-off between selectivity and adsorption capacity for gas separation. Chem 7:3085–3098. https://doi.org/10.1016/j.chempr.2021.07.007
Langhammer D, Kullgren J, Mitev P, Österlund L (2018) SO2 adsorption on rutile TiO2(110): an infrared reflection-absorption spectroscopy and density functional theory study. Surf Sci 677:46–51. https://doi.org/10.1016/j.susc.2018.05.016
Langhammer D, Thyr J, Österlund L (2019) Surface properties of reduced and stoichiometric TiO2 as probed by SO2 adsorption. J Phys Chem C 123:24549–24557. https://doi.org/10.1021/acs.jpcc.9b05805
Li J, Kobayashi N, Hu Y (2008) The activated coke preparation for SO2 adsorption by using flue gas from coal power plant. Chem Eng Process 47:118–127. https://doi.org/10.1016/j.cep.2007.08.001
Li Z, Liu Y, Wang H et al (2018) A numerical modelling study of SO2 adsorption on activated carbons with new rate equations. Chem Eng J 353:858–866. https://doi.org/10.1016/j.cej.2018.07.119
Li H, Liu S, Yang J et al (2020) Role of SO2 and H2O in the mercury adsorption on ceria surface: a DFT study. Fuel 260:116289. https://doi.org/10.1016/j.fuel.2019.116289
Lo JMH, Ziegler T, Clark PD (2010) SO2 adsorption and transformations on γ-Al2O3 surfaces: a density functional theory study. J Phys Chem C 114:10444–10454. https://doi.org/10.1021/jp910895g
López D, Buitrago R, Sepúlveda-Escribano A et al (2008) Low temperature catalytic adsorption of SO2 on activated carbon. J Phys Chem C 112:15335–15340. https://doi.org/10.1021/jp802809c
Maheswari C, Krishnamurthy K, Parameshwaran R (2014) Modeling and experimental analysis of packed column for SO2 emission control process. Atmos Pollut Res 5:464–470. https://doi.org/10.5094/apr.2014.054
Muzio LJ, Often GR (1987) Assessment of dry sorbent emission control technologies Part I fundamental processes. JAPCA 37:642–654. https://doi.org/10.1080/08940630.1987.10466251
Mykola S, Bandosz TJ (2010) Effects of surface features on adsorption of SO2 on graphite oxide/Zr(OH)4 composites. J Phys Chem C 114:14552–14560. https://doi.org/10.1021/jp1051479
Ohashi N, Yoshizawa K, Endou A et al (2001) Adsorption properties of SO2 on ultrafine precious metal particles studied using density functional calculation. Appl Surf Sci 177:180–188. https://doi.org/10.1016/s0169-4332(01)00198-2
Pandey NK, Velavendan P, Geetha R et al (1998) Adsorption kinetics and breakthrough behaviour of Tri-n-butyl phosphate on Amberlite XAD-4 Resin. J Nucl Sci Technol 35:370–378. https://doi.org/10.1080/18811248.1998.9733874
Pedrolo DRS, de Menezes Quines LK, de Souza G, Marcilio NR (2017) Synthesis of zeolites from Brazilian coal ash and its application in SO2 adsorption. J Environ Chem Eng 5:4788–4794. https://doi.org/10.1016/j.jece.2017.09.015
Punyawudho K, Ma S, Zee V, Monnier JR (2011) Effect of O2 on the adsorption of SO2 on carbon-supported Pt electrocatalysts. Langmuir 27:7524–7530. https://doi.org/10.1021/la2000377
Qie Z, Sun F, Gao J et al (2020) Enhanced SO2 fluidized adsorption dynamic by hierarchically porous activated coke. J Energy Inst 93:802–810. https://doi.org/10.1016/j.joei.2019.05.002
Qin H, Feng C, Luan X, Yang D (2018) First-principles investigation of adsorption behaviors of small molecules on penta-graphene. Nanoscale Res Lett 13:1–7. https://doi.org/10.1186/s11671-018-2687-y
Radoiu MT, Martin DI, Calinescu I (2003) Emission control of SO2 and NOx by irradiation methods. J Hazard Mater 97:145–158. https://doi.org/10.1016/s0304-3894(02)00256-x
Raymundo-Piñero E, Cazorla-Amorós D, Linares-Solano A (2001) Temperature programmed desorption study on the mechanism of SO2 oxidation by activated carbon and activated carbon fibres. Carbon 39:231–242. https://doi.org/10.1016/s0008-6223(00)00119-6
Ren J, Kong W, Ni J (2019) The potential application of BAs for a gas sensor for detecting SO2 gas molecule: a DFT study. Nanoscale Res Lett 14:133. https://doi.org/10.1186/s11671-019-2972-4
Rojas-Mayorga CK, Aguayo-Villarreal IA, Moreno-Pérez J et al (2021) Influence of calcium species on SO2 adsorption capacity of a novel carbonaceous materials and their ANN modeling. J Environ Chem Eng 9:104810. https://doi.org/10.1016/j.jece.2020.104810
Shi L, Yang K, Zhao Q et al (2015) Characterization and mechanisms of H2S and SO2 adsorption by activated carbon. Energy Fuels 29:6678–6685. https://doi.org/10.1021/acs.energyfuels.5b01696
Silas K, Ghani WAWAK, Choong TSY, Rashid U (2018) Breakthrough studies of Co3O4 supported activated carbon monolith for simultaneous SO2/NO removal from flue gas. Fuel Process Technol 180:155–165. https://doi.org/10.1016/j.fuproc.2018.08.018
Skorjanc T, Shetty D, Trabolsi A (2021) Pollutant removal with organic macrocycle-based covalent organic polymers and frameworks. Chem 7:882–918. https://doi.org/10.1016/j.chempr.2021.01.002
Streeter JL (2016) Adoption of SO2 emission control technologies—an application of survival analysis. Energy Policy 90:16–23. https://doi.org/10.1016/j.enpol.2015.11.035
Sumathi S, Bhatia S, Lee KT, Mohamed AR (2010) Adsorption isotherm models and properties of SO2 and NO removal by palm shell activated carbon supported with cerium (Ce/PSAC). Chem Eng J 162:194–200. https://doi.org/10.1016/j.cej.2010.05.028
Sun F, Gao J, Zhu Y, Qin Y (2011) Mechanism of SO2 adsorption and desorption on commercial activated coke. Korean J Chem Eng 28:2218–2225. https://doi.org/10.1007/s11814-011-0078-5
Sun L, Ning P, Zhao X et al (2021) Competitive adsorption and reaction mechanism on simultaneous catalytic removal of SO2, NO and HgO over CuO: Experimental and theoretical studies. Chem Eng J 412:128752. https://doi.org/10.1016/j.cej.2021.128752
Taylor MR, Rubin ES, Hounshell DA (2005) Control of SO2 emissions from power plants: a case of induced technological innovation in the US. Technol Forecast Soc Chang 72:697–718. https://doi.org/10.1016/j.techfore.2004.11.001
Volzone C, Ortiga J (2011) SO2 gas adsorption by modified kaolin clays: Influence of previous heating and time acid treatments. J Environ Manage 92:2590–2595. https://doi.org/10.1016/j.jenvman.2011.05.031
Wang J, Zhu Z, Li C (1999) Pathway of the cycle between the oxidative adsorption of SO2 and the reductive decomposition of sulfate on the MgAl2−xFexO4 catalyst. J Mol Catal a: Chem 139:31–41. https://doi.org/10.1016/s1381-1169(98)00186-1
Wang C, Liu H, Zhang Y et al (2018) Review of arsenic behavior during coal combustion: volatilization, transformation, emission and removal technologies. Prog Energy Combust Sci 68:1–28. https://doi.org/10.1016/j.pecs.2018.04.001
Wang J, Yi H, Tang X et al (2020) Simultaneous removal of SO2 and NOx by catalytic adsorption using γ-Al2O3 under the irradiation of non-thermal plasma: competitiveness, kinetic, and equilibrium. Chem Eng J 384:123334. https://doi.org/10.1016/j.cej.2019.123334
Wang S, Xu S, Gao S et al (2021) Simultaneous removal of SO2 and NOx from flue gas by low-temperature adsorption over activated carbon. Sci Rep 11:11003. https://doi.org/10.1038/s41598-021-90532-9
Wei H, Gui Y, Kang J et al (2018) A DFT study on the adsorption of H2S and SO2 on Ni doped MoS2 monolayer. Nanomaterials 8:646. https://doi.org/10.3390/nano8090646
Wilburn MS, Epling WS (2017) SO2 adsorption and desorption characteristics of Pd and Pt catalysts: precious metal crystallite size dependence. Appl Catal A 534:85–93. https://doi.org/10.1016/j.apcata.2017.01.015
Wilburn MS, Epling WS (2019) SO2 adsorption and desorption characteristics of bimetallic Pd-Pt catalysts: Pd: Pt ratio dependency. Catal Today 320:11–19. https://doi.org/10.1016/j.cattod.2017.08.054
Wu D, Sun C, Dutta PK, Winston Ho WS (2017) SO2 interference on separation performance of amine-containing facilitated transport membranes for CO2 capture from flue gas. J Membr Sci 534:33–45. https://doi.org/10.1016/j.memsci.2017.04.003
Yang W, Zhang J, Ma Q et al (2017) Heterogeneous reaction of SO2 on manganese oxides: the effect of crystal structure and relative humidity. Sci Rep 7:4550. https://doi.org/10.1038/s41598-017-04551-6
Yang K, Yi H, Tang X et al (2019) Reducing the competitive adsorption between SO2 and NO by Al2O3@TiO2 core-shell structure adsorbent. Chem Eng J 364:420–427. https://doi.org/10.1016/j.cej.2019.02.009
Ye X, Jiang X, Chen L et al (2020) Effect of manganese dioxide crystal structure on adsorption of SO2 by DFT and experimental study. Appl Surf Sci 521:146477. https://doi.org/10.1016/j.apsusc.2020.146477
Yi H, Wang Z, Liu H et al (2014) Adsorption of SO2, NO, and CO2 on activated carbons: equilibrium and thermodynamics. J Chem Eng Data 59:1556–1563. https://doi.org/10.1021/je4011135
Yi H, Ma C, Tang X et al (2019) Synthesis of MgO@CeO2-MnOx core shell structural adsorbent and its application in reducing the competitive adsorption of SO2 and NOx in coal-fired flue gas. Chem Eng J 372:129–140. https://doi.org/10.1016/j.cej.2019.04.120
Yogi R, Jaiswal NK, Srivastava P (2020) First-principles study of sensing SO2 adsorption on III–V nitride nanoribbons. Mater Chem Phys 242:122437. https://doi.org/10.1016/j.matchemphys.2019.122437
Zhang T, Li J, Yu S, Wang Y (2014) Preparation and characterization of a new desulfurizer and its performance on removal of SO2. J Geosci Environ Prot 02:68–76. https://doi.org/10.4236/gep.2014.22011
Zhang K, Ren S, Yang X et al (2017) Efficient absorption of low-concentration SO2 in simulated flue gas by functional deep eutectic solvents based on imidazole and its derivatives. Chem Eng J 327:128–134. https://doi.org/10.1016/j.cej.2017.06.081
Zhang Y, Chen Z, Liu X et al (2019) Efficient SO2 removal using a microporous metal-organic framework with molecular sieving effect. Ind Eng Chem Res 59:874–882. https://doi.org/10.1021/acs.iecr.9b06040
Zhao L, Li X, Hao C, Raston CL (2012) SO2 adsorption and transformation on calcined NiAl hydrotalcite-like compounds surfaces: an in situ FTIR and DFT study. Appl Catal B 117–118:339–345. https://doi.org/10.1016/j.apcatb.2012.01.034
Zhi Y, Zhou Y, Su W et al (2011) Selective adsorption of SO2 from flue gas on triethanolamine-modified large pore SBA-15. Ind Eng Chem Res 50:8698–8702. https://doi.org/10.1021/ie2004658
Zhu JL, Wang YH, Zhang JC, Ma RY (2005) Experimental investigation of adsorption of NO and SO2 on modified activated carbon sorbent from flue gases. Energy Convers Manage 46:2173–2184. https://doi.org/10.1016/j.enconman.2004.10.011
Zhu X, Li S, Shi Y, Cai N (2019) Recent advances in elevated-temperature pressure swing adsorption for carbon capture and hydrogen production. Prog Energy Combust Sci 75:100784. https://doi.org/10.1016/j.pecs.2019.100784
Zou B, Peng F, Wan N et al (2014) Sulfur dioxide exposure and environmental justice: a multi–scale and source–specific perspective. Atmos Pollut Res 5:491–499. https://doi.org/10.5094/APR.2014.058
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
A. carried the experiment, analysed, optimized, and wrote the paper. B. reviewed, analysed, suggested, and advised
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jeyapaul, A.S., Ganesapillai, M. A sustainable solution: mitigating sulphur dioxide emissions through adsorption on chemically modified iron oxide nanoparticles. Clean Techn Environ Policy (2024). https://doi.org/10.1007/s10098-024-02807-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10098-024-02807-0