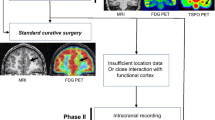
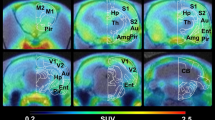
Abstract
Increasing evidence indicate that neuroinflammation triggered by glial cells plays a significant role in epileptogenesis. To this effect, the overexpression of translocator protein 18 kDa (TSPO) in activated microglia and astrocytes has been identified as an inflammatory biomarker in epilepsy. It is now possible to quantify neuroinflammation using non-invasive positron emission tomography (PET) imaging of TSPO. With the advancement of radiotracers, TSPO PET has become an innovative tool in elucidating the "neuroinflammatory machinery" of drug-resistant epilepsy. Furthermore, TSPO PET has demonstrated potential in detecting MRI-negative epileptogenic zones (EZ) and provided an innovative perspective in epileptic medical treatment. This manuscript presents a comprehensive exploration of the neuroinflammatory mechanisms of epilepsy, alongside a thorough review of TSPO PET studies conducted in clinical and preclinical settings. The primary objective is to deepen our understanding of epilepsy progression and to establish TSPO PET as an effective monitoring tool for treatment efficacy.

Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ngugi AK et al (2010) Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia 51(5):883–890
Keezer MR, Sisodiya SM, Sander JW (2016) Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol 15(1):106–115
Weaver DF, Pohlmann-Eden B (2013) Pharmacoresistant epilepsy: unmet needs in solving the puzzle(s). Epilepsia 54(Suppl 2):80–85
Kalilani L et al (2018) The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsia 59(12):2179–2193
Brackhan M et al (2018) [(18) F]GE180 positron emission tomographic imaging indicates a potential double-hit insult in the intrahippocampal kainate mouse model of temporal lobe epilepsy. Epilepsia 59(3):617–626
Nguyen DL et al (2018) Longitudinal positron emission tomography imaging of glial cell activation in a mouse model of mesial temporal lobe epilepsy: Toward identification of optimal treatment windows. Epilepsia 59(6):1234–1244
Zhang PF, Gao F (2022) Neuroinflammation in Parkinson’s disease: a meta-analysis of PET imaging studies. J Neurol 269(5):2304–2314
YankamNjiwa J et al (2017) Quantitative longitudinal imaging of activated microglia as a marker of inflammation in the pilocarpine rat model of epilepsy using [(11)C]-( R)-PK11195 PET and MRI. J Cereb Blood Flow Metab 37(4):1251–1263
Weidner LD et al (2018) The expression of inflammatory markers and their potential influence on efflux transporters in drug-resistant mesial temporal lobe epilepsy tissue. Epilepsia 59(8):1507–1517
Russmann V et al (2017) Identification of brain regions predicting epileptogenesis by serial [(18)F]GE-180 positron emission tomography imaging of neuroinflammation in a rat model of temporal lobe epilepsy. Neuroimage Clin 15:35–44
Bertoglio D et al (2017) Non-invasive PET imaging of brain inflammation at disease onset predicts spontaneous recurrent seizures and reflects comorbidities. Brain Behav Immun 61:69–79
Butler T et al (2013) Imaging inflammation in a patient with epilepsy due to focal cortical dysplasia. J Neuroimaging 23(1):129–131
Wolf BJ et al (2020) TSPO PET Identifies Different Anti-inflammatory Minocycline Treatment Response in Two Rodent Models of Epileptogenesis. Neurotherapeutics 17(3):1228–1238
Vezzani A, Friedman A, Dingledine RJ (2013) The role of inflammation in epileptogenesis. Neuropharmacology 69:16–24
Müller S et al (2001) New EMBO members’ review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J 20(16):4337–4340
Balosso S et al (2014) Disulfide-containing high mobility group box-1 promotes N-methyl-D-aspartate receptor function and excitotoxicity by activating Toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxid Redox Signal 21(12):1726–1740
Sharma R et al (2019) Neuroinflammation in Post-Traumatic Epilepsy: Pathophysiology and Tractable Therapeutic Targets. Brain Sci 9:11
Rana A, Musto AE (2018) The role of inflammation in the development of epilepsy. J Neuroinflammation 15(1):144
Terrone G et al (2020) Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology 167:107742
Hodges SL, Lugo JN (2020) Therapeutic role of targeting mTOR signaling and neuroinflammation in epilepsy. Epilepsy Res 161:106282
Ostendorf AP, Wong M (2015) mTOR inhibition in epilepsy: rationale and clinical perspectives. CNS Drugs 29(2):91–99
Oh YJ, Francis JW, Markelonis GJ, Oh TH (1992) Interleukin-1-beta and tumor necrosis factor-alpha increase peripheraltype benzodiazepine binding sites in cultured polygonal astrocytes. J Neurochem. 58(6):2131–8 https://doi.org/10.1111/j.1471-4159.1992.tb10955.x
Nomura M et al (2021) Peripheral benzodiazepine receptor/18 kDa translocator protein positron emission tomography imaging in a rat model of acute brain injury. Ann Nucl Med 35(1):8–16
Meng Y et al (2020) Downregulation of TSPO expression inhibits oxidative stress and maintains mitochondrial homeostasis in cardiomyocytes subjected to anoxia/reoxygenation injury. Biomed Pharmacother 121:109588
Biswas L et al (2018) TSPO Ligands Promote Cholesterol Efflux and Suppress Oxidative Stress and Inflammation in Choroidal Endothelial Cells. Int J Mol Sci 19:12
Kumar A et al (2008) Epilepsy Surgery in a Case of Encephalitis: Use of 11C-PK11195 Positron Emission Tomography. Pediatr Neurol 38(6):439–442
Bogdanović RM et al (2014) (R)-[11C]PK11195 brain uptake as a biomarker of inflammation and antiepileptic drug resistance: Evaluation in a rat epilepsy model. Neuropharmacology 85:104–112
Passamonti L et al (2018) [(11)C]PK11195 binding in Alzheimer disease and progressive supranuclear palsy. Neurology 90(22):e1989–e1996
Yokokura M et al (2017) Depiction of microglial activation in aging and dementia: Positron emission tomography with [(11)C]DPA713 versus [(11)C]( R)PK11195. J Cereb Blood Flow Metab 37(3):877–889
Vallez Garcia D et al (2016) Three Month Follow-Up of Rat Mild Traumatic Brain Injury: A Combined [(18)F]FDG and [(11)C]PK11195 Positron Emission Study. J Neurotrauma 33(20):1855–1865
Zhang L et al (2021) Recent developments on PET radiotracers for TSPO and their applications in neuroimaging. Acta Pharmaceutica Sinica B 11(2):373–393
Dickstein LP et al (2019) Neuroinflammation in neocortical epilepsy measured by PET imaging of translocator protein. Epilepsia 60(6):1248–1254
Kagitani-Shimono K et al (2022) Extension of microglial activation is associated with epilepsy and cognitive dysfunction in Tuberous sclerosis complex: A TSPO-PET study. Neuroimage Clin 37:103288
Dedeurwaerdere S et al (2012) PET imaging of brain inflammation during early epileptogenesis in a rat model of temporal lobe epilepsy. EJNMMI Res 2(1):60
Fan Z et al (2015) Can Studies of Neuroinflammation in a TSPO Genetic Subgroup (HAB or MAB) Be Applied to the Entire AD Cohort? J Nucl Med 56(5):707–713
Guo Q et al (2012) Identifying improved TSPO PET imaging probes through biomathematics: the impact of multiple TSPO binding sites in vivo. Neuroimage 60(2):902–910
Unterrainer M et al (2018) TSPO PET with [(18)F]GE-180 sensitively detects focal neuroinflammation in patients with relapsing-remitting multiple sclerosis. Eur J Nucl Med Mol Imaging 45(8):1423–1431
Fujita M et al (2017) Comparison of four (11)C-labeled PET ligands to quantify translocator protein 18 kDa (TSPO) in human brain: (R)-PK11195, PBR28, DPA-713, and ER176-based on recent publications that measured specific-to-non-displaceable ratios. EJNMMI Res 7(1):84
Scott G et al (2017) Microglial positron emission tomography (PET) imaging in epilepsy: Applications, opportunities and pitfalls. Seizure 44:42–47
Vezzani A, Balosso S, Ravizza T (2008) The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun 22(6):797–803. https://doi.org/10.1016/j.bbi.2008.03.009
Potschka H (2010) Modulating P-glycoprotein regulation: future perspectives for pharmacoresistant epilepsies? Epilepsia 51(8):1333–47. https://doi.org/10.1111/j.1528-1167.2010.02585.x
Vezzani A, Balosso S, Ravizza T (2008) The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun 22(6):797–803
Potschka H (2010) Modulating P-glycoprotein regulation: future perspectives for pharmacoresistant epilepsies? Epilepsia 51(8):1333–1347
Sierra A, Grohn O, Pitkanen A (2015) Imaging microstructural damage and plasticity in the hippocampus during epileptogenesis. Neuroscience 309:162–172
Amhaoul H et al (2015) Brain inflammation in a chronic epilepsy model: Evolving pattern of the translocator protein during epileptogenesis. Neurobiol Dis 82:526–539
Brackhan M et al (2016) Serial Quantitative TSPO-Targeted PET Reveals Peak Microglial Activation up to 2 Weeks After an Epileptogenic Brain Insult. J Nucl Med 57(8):1302–1308
Bertoglio D et al (2021) TSPO PET upregulation predicts epileptic phenotype at disease onset independently from chronic TSPO expression in a rat model of temporal lobe epilepsy. Neuroimage Clin 31:102701
Casadei CH et al (2020) All-cause mortality and SUDEP in a surgical epilepsy population. Epilepsy Behav 108:107093
Sperling MR et al (2016) A reappraisal of mortality after epilepsy surgery. Neurology 86(21):1938–1944
Gershen LD et al (2015) Neuroinflammation in temporal lobe epilepsy measured using positron emission tomographic imaging of translocator protein. JAMA Neurol 72(8):882–8. https://doi.org/10.1001/jamaneurol.2015.0941
Kagitani-Shimono K et al (2021) Clinical evaluation of neuroinflammation in child-onset focal epilepsy: a translocator protein PET study. J Neuroinflammation 18:1
Gershen LD et al (2015) Neuroinflammation in Temporal Lobe Epilepsy Measured Using Positron Emission Tomographic Imaging of Translocator Protein. JAMA Neurol 72(8):882–888
Ikawa M et al (2017) 11C-ER176, a Radioligand for 18-kDa Translocator Protein, Has Adequate Sensitivity to Robustly Image All Three Affinity Genotypes in Human Brain. J Nucl Med 58(2):320–325
Bouilleret V, Dedeurwaerdere S (2021) What value can TSPO PET bring for epilepsy treatment? Eur J Nucl Med Mol Imaging 49(1):221–233
Luu TG, Kim HK (2022) (18)F-Radiolabeled Translocator Protein (TSPO) PET Tracers: Recent Development of TSPO Radioligands and Their Application to PET Study. Pharmaceutics 14:11
Butler T et al (2016) Transient and chronic seizure-induced inflammation in human focal epilepsy. Epilepsia 57(9):e191–e194
Russmann V et al (2016) Minocycline fails to exert antiepileptogenic effects in a rat status epilepticus model. Eur J Pharmacol 771:29–39
Vezzani A (2015) Anti-inflammatory drugs in epilepsy: does it impact epileptogenesis? Expert Opin Drug Saf 14(4):583–592
Singh T et al (2022) Reconnoitering the transformative journey of minocycline from an antibiotic to an antiepileptic drug. Life Sci 293:120346. https://doi.org/10.1016/j.lfs.2022.120346
Godfred RM et al (2013) Does aspirin use make it harder to collect seizures during elective video-EEG telemetry? Epilepsy Behav 27(1):115–117
Singh T et al (2022) Reconnoitering the transformative journey of minocycline from an antibiotic to an antiepileptic drug. Life Sci 293:120346
Koneval Z et al (2018) Lamotrigine-resistant corneal-kindled mice: A model of pharmacoresistant partial epilepsy for moderate-throughput drug discovery. Epilepsia 59(6):1245–1256
Wang N et al (2015) Minocycline inhibits brain inflammation and attenuates spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neuroscience 287:144–156
Wang DD, Englot DJ, Garcia PA, Lawton MT, Young WL (2012) Minocycline- and tetracycline-class antibiotics are protective against partial seizures in vivo. Epilepsy Behav 24(3):314–8. https://doi.org/10.1016/j.yebeh.2012.03.035
Vezzani A, Balosso S, Ravizza T (2019) Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol 15(8):459–472
Wang DD et al (2012) Minocycline- and tetracycline-class antibiotics are protective against partial seizures in vivo. Epilepsy Behav 24(3):314–318
DiSabato DJ, Quan N, Godbout JP (2016) Neuroinflammation: the devil is in the details. J Neurochem 139(S2):136–153
Benson MJ, Manzanero S, Borges K (2015) Complex alterations in microglial M1/M2 markers during the development of epilepsy in two mouse models. Epilepsia 56(6):895–905
Ali I, Chugh D, Ekdahl CT (2015) Role of fractalkine-CX3CR1 pathway in seizure-induced microglial activation, neurodegeneration, and neuroblast production in the adult rat brain. Neurobiol Dis 74:194–203
Wu P et al (2022) P2X7 receptor induces microglia polarization to the M1 phenotype in cancer-induced bone pain rat models. Mol Pain 18:17448069211060962
Hirvonen J et al (2012) Increased in vivo expression of an inflammatory marker in temporal lobe epilepsy. J Nucl Med 53(2):234–240
Kaneko KI et al (2022) [(18)F]DPA-714 PET imaging for the quantitative evaluation of early spatiotemporal changes of neuroinflammation in rat brain following status epilepticus. Eur J Nucl Med Mol Imaging 49(7):2265–2275
Acknowledgements
We are grateful for funding from National Key Research and Development Program of China, Grant No. 2022YFC2503804, from National Natural Science Foundation of China, Grant Nos. 82071461, 82271503, and from Natural Science Foundation of Hunan Province, Grant No. 2021JJ31060. We further thank anonymous reviewers for constructive feedback.
Author information
Authors and Affiliations
Contributions
All author contributed to the writing of this manuscript. They have seen and approved the final version of the manuscript being submitted and warrant that the article is original work which hasn't received prior publication and isn't under consideration for publication elsewhere.
Corresponding authors
Ethics declarations
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this manuscript is consistent with those guidelines.
Conflict of interest
The authors declare that they have no conficts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li Qin and Ling **ao contributed equally to this work as co-first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, L., **ao, L., Zhu, H. et al. Translocator protein (18 kDa) positron emission tomography imaging as a biomarker of neuroinflammation in epilepsy. Neurol Sci (2024). https://doi.org/10.1007/s10072-024-07648-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10072-024-07648-9