Abstract
Objective
This study aimed to assess the diagnostic potential of the Antibody concentration ratio in identifying treatment-refractory myasthenia gravis (MG).
Methods
A retrospective analysis was conducted on 116 MG patients who underwent antibody detection at least twice between June 1, 2015, and June 1, 2023. Demographic and clinical characteristics were collated to ascertain their association with refractory MG. The Antibody Concentration Ratio was applied to determine treatment response, using the International Consensus Guidance criteria as the reference standard. The area under nonparametric receiver operating characteristic curve (AUC), sensitivity, specificity, and accuracy were calculated to assess the diagnostic efficacy of the Antibody concentration ratio following consecutive immunotherapy relative to initial antibody concentrations for refractory MG.
Results
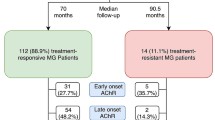
19 out of 116 patients were unequivocally diagnosed with refractory MG. A significant correlation was found between the Antibody Concentration Ratio and refractory MG status in treatment-refractory and treatment-responsive patients. Subsequently, the AUC demonstrated the robust diagnostic capability of the Antibody concentration ratio for refractory MG, with an AUC of 0.8709 (95% CI: 0.7995–0.9422, p < 0.0001). The optimal cut-off value stood at 0.8903, exhibiting a sensitivity of 94.74% (95% CI: 75.36%-99.73%), a specificity of 68.04% (95% CI: 58.23%-76.48%), and accuracy of 72.41% (95% CI: 64.28%-80.54%).
Conclusion
Elevated Antibody Concentration Ratio is intrinsically linked with refractory MG and exhibits potential as an diagnostic biomarker for the condition.



Similar content being viewed by others
Data Availability
Data that support the findings of this study is availability. https://figshare.com/. https://doi.org/10.6084/m9.figshare.25287790.
Code availability
Not applicable.
Abbreviations
- MG:
-
Myasthenia gravis
- ROC:
-
Receiver operating characteristic
- AChR:
-
Acetylcholine receptor
- MuSK:
-
Muscle-specific tyrosine kinase
- IVIG:
-
Intravenous immunoglobulin
- PLEX:
-
Plasma exchange
- QMGs:
-
The quantitative myasthenia gravis scores
- MG-ADLs:
-
MG activities of daily living scores
- ICG:
-
International Consensus Guidance
- PASS:
-
Patient-acceptable symptom status
- MGFA:
-
MG Foundation of America
- AUC:
-
Area under the ROC curve
References
Keesey JC (2004) Clinical evaluation and management of myasthenia gravis. Muscle Nerve 29(4):484–505
Lindstrom JM, Seybold ME, Lennon VA, Whittingham S, Duane DD (1976) Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology 26(11):1054–9
Evoli A, Alboini PE, Damato V et al (2018) Myasthenia gravis with antibodies to MuSK: an update. Ann N Y Acad Sci 1412(1):82–89
Berrih-Aknin S, Frenkian-Cuvelier M, Eymard B (2014) Diagnostic and clinical classification of autoimmune myasthenia gravis. J Autoimmun 48–49:143–148
Cortés-Vicente E, Álvarez-Velasco R, Pla-Junca F et al (2022) Drug-refractory myasthenia gravis: Clinical characteristics, treatments, and outcome. Ann Clin Transl Neurol 9(2):122–131
Dalakas MC (2019) Immunotherapy in myasthenia gravis in the era of biologics. Nat Rev Neurol 15(2):113–124
Rath J, Brunner I, Tomschik M et al (2020) Frequency and clinical features of treatment-refractory myasthenia gravis. J Neurol 267(4):1004–1011
Mantegazza R, Antozzi C (2018) When myasthenia gravis is deemed refractory: clinical signposts and treatment strategies. Ther Adv Neurol Disord 11:1756285617749134
Veltsista D, Kefalopoulou Z, Tzartos J, Chroni E (2022) Autoantibody profile in myasthenia gravis patients with a refractory phase. Muscle Nerve 65(5):607–611
Kojima Y, Uzawa A, Ozawa Y et al (2021) Rate of change in acetylcholine receptor antibody levels predicts myasthenia gravis outcome. J Neurol Neurosurg Psychiatry 92(9):963–968
Jeong S, Noh Y, Oh IS, Hong YH, Shin JY (2021) Survival, Prognosis, and Clinical Feature of Refractory Myasthenia Gravis: a 15-year Nationwide Cohort Study. J Korean Med Sci 36(39):e242
Sanders DB, Burns TM, Cutter GR, Massey JM, Juel VC, Hobson-Webb L (2014) Does change in acetylcholine receptor antibody level correlate with clinical change in myasthenia gravis. Muscle Nerve 49(4):483–486
Oosterhuis HJ, Limburg PC, Hummel-Tappel E, The TH (1983) Anti-acetylcholine receptor antibodies in myasthenia gravis. Part 2. Clinical and serological follow-up of individual patients. J Neurol Sci 58(3):371–85
Heldal AT, Eide GE, Romi F, Owe JF, Gilhus NE (2014) Repeated acetylcholine receptor antibody-concentrations and association to clinical myasthenia gravis development. PLoS ONE 9(12):e114060
Sanders DB, Wolfe GI, Benatar M et al (2016) International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 87(4):419–425
Tran C, Biswas A, Mendoza M, Katzberg H, Bril V, Barnett C (2021) Performance of different criteria for refractory myasthenia gravis. Eur J Neurol 28(4):1375–1384
Katzberg HD, Barnett C, Merkies IS, Bril V (2014) Minimal clinically important difference in myasthenia gravis: outcomes from a randomized trial. Muscle Nerve 49(5):661–665
Kanai T, Uzawa A, Kawaguchi N et al (2017) Adequate tacrolimus concentration for myasthenia gravis treatment. Eur J Neurol 24(2):270–275
Mendoza M, Tran C, Bril V, Katzberg HD, Barnett C (2020) Patient-acceptable symptom states in myasthenia gravis. Neurology 95(12):e1617–e1628
Jaretzki A 3rd, Barohn RJ, Ernstoff RM et al (2000) Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 55(1):16–23
Schneider-Gold C, Hagenacker T, Melzer N, Ruck T (2019) Understanding the burden of refractory myasthenia gravis. Ther Adv Neurol Disord 12:1756286419832242
Narayanaswami P, Sanders DB, Wolfe G et al (2021) International Consensus Guidance for Management of Myasthenia Gravis: 2020 Update. Neurology 96(3):114–122
Álvarez-Velasco R, Gutiérrez-Gutiérrez G, Trujillo JC et al (2021) Clinical characteristics and outcomes of thymoma-associated myasthenia gravis. Eur J Neurol 28(6):2083–2091
Newsom-Davis J, Pinching AJ, Vincent A, Wilson SG (1978) Function of circulating antibody to acetylcholine receptor in myasthenia gravis: investigation by plasma exchange. Neurology 28(3):266–272
Usmani A, Kwan L, Wahib-Khalil D, Trivedi J, Nations S, Sarode R (2019) Excellent response to therapeutic plasma exchange in myasthenia gravis patients irrespective of antibody status. J Clin Apher 34(4):416–422
Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren J (2019) Myasthenia gravis Nat Rev Dis Primers 5(1):30
Hewer R, Matthews I, Chen S, McGrath V, Evans M, Roberts E et al (2006) A sensitive non-isotopic assay for acetylcholine receptor autoantibodies. Clin Chim Acta 364(1–2):159–166
Schneider-Gold C, Reinacher-Schick A, Ellrichmann G, Gold R (2017) Bortezomib in severe MuSK-antibody positive myasthenia gravis: first clinical experience. Ther Adv Neurol Disord 10(10):339–341
Hill ME, Shiono H, Newsom-Davis J, Willcox N (2008) The myasthenia gravis thymus: a rare source of human autoantibody-secreting plasma cells for testing potential therapeutics. J Neuroimmunol 201–202:50–56
Dalakas MC (2020) Progress in the therapy of myasthenia gravis: getting closer to effective targeted immunotherapies. Curr Opin Neurol 33(5):545–552
Acknowledgements
This research was supported by the National Natural Science Foundation of China (grant number: 82171399).
Author information
Authors and Affiliations
Contributions
Huan Yang: conception, study design, revision of the manuscript; Yi Li: manuscript writing, interpretation of the results and collection of data; Shumei Yang: data collection and manuscript revision; **aohua Dong, Fei Jiang, Kangzhi Chen, Qian Zhou, Haobin Cai participated in the collection of data.
Corresponding author
Ethics declarations
Conflicts of interest
None of the authors has any conflict of interest to disclose.
Ethics approval
The study complied with the Declaration of Helsinki. All participants were informed and signed the consent form for the study and was approved by the Medical Ethics Committee of the Hunan Medical Science Research Institute of **angya Hospital (202103207).
Consent to participate
All participants were informed and signed the consent form for the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Yang, S., Dong, X. et al. Diagnostic value of antibody concentration ratio for treatment-refractory myasthenia gravis. Neurol Sci (2024). https://doi.org/10.1007/s10072-024-07601-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10072-024-07601-w