Abstract
Background
Studies targeting amyloid-ß in patients with Alzheimer’s disease (AD) have conflicting results and early initiation of therapy may yield better outcomes.
Methods
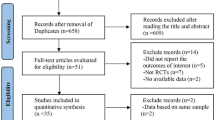
We systematically searched PubMed, Embase, Cochrane Library, and Clinicaltrials.gov for randomized trials comparing monoclonal antibodies (mAbs) with placebo in MCI or mild dementia due to AD.
Results
Nineteen studies comprising 15,275 patients were included. In patients with early AD, mAbs reduced the rate of decline, in both the Clinical Dementia Rating Scale, the sum of boxes (CDR-SB; MD −0.30; 95% CI −0.42,−0.19; p < 0.01), and the Alzheimer’s Disease Assessment Scale, cognitive subscore (ADAS-cog; SMD −0.80; 95% CI −10.25,−0.35; p < 0.01). The results were similar between clinical stages for CDR-SB (MCI, MD −0.19; 95% CI −0.35,−0.03; p = 0.02; mild dementia, MD −0.45; 95% CI −0.65,−0.25; p < 0.01; subgroup differences, p = 0.13), as well as for ADAS-Cog (MCI, SMD −0.83; 95% CI −1.49,−0.17; p = 0.01; mild dementia, SMD −0.69; 95% CI −1.32 to −0.05; p = 0.03; subgroup differences, p = 0.47). The risk of amyloid-related imaging abnormalities (ARIA) was significantly higher in patients taking mAbs, including ARIA-edema (RR 7.7; 95% CI 4.60 to 13.00; p < 0.01), ARIA-hemorrhage (RR 1.8; 95% CI 1.22 to 2.59; p < 0.01), and symptomatic or serious ARIA (RR 14.1; 95% CI 7.30 to 27.14; p < 0.01).
Conclusion
Anti-amyloid-ß mAbs attenuate cognitive and functional decline compared with placebo in early AD; whether the magnitude of this effect is clinically important remains uncertain, especially relative to the safety profile of these medications. Starting immunotherapy in patients with MCI was not significantly different than starting in the mild dementia stage.
PROSPERO registry
CRD42023430698




Similar content being viewed by others
Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.
References
Tahami Monfared AA, Byrnes MJ, White LA, Zhang Q (2022) Alzheimer’s disease: epidemiology and clinical progression. Neurol Ther 11:553–569. https://doi.org/10.1007/s40120-022-00338-8
DeTure MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14:32. https://doi.org/10.1186/s13024-019-0333-5
Kocahan S, Doğan Z (2017) Mechanisms of Alzheimer’s disease pathogenesis and prevention: the brain, neural pathology, N-methyl-D-aspartate receptors, Tau Protein and Other Risk Factors. Clin Psychopharmacol Neurosci 15:1–8. https://doi.org/10.9758/cpn.2017.15.1.1
Heneka MT, Carson MJ, Khoury JE et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405. https://doi.org/10.1016/S1474-4422(15)70016-5
Mitchell AJ, Shiri-Feshki M (2009) Rate of progression of mild cognitive impairment to dementia - meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand 119:252–265. https://doi.org/10.1111/j.1600-0447.2008.01326.x
Frank L, Lloyd A, Flynn JA et al (2006) Impact of cognitive impairment on mild dementia patients and mild cognitive impairment patients and their informants. Int Psychogeriatr 18:151–162. https://doi.org/10.1017/S1041610205002450
Rice DP, Fillit HM, Max W et al (2001) Prevalence, costs, and treatment of Alzheimer’s disease and related dementia: a managed care perspective. Am J Manag Care 7:809–818
Lin P, Neumann PJ (2013) The economics of mild cognitive impairment. Alzheimers Dement 9:58–62. https://doi.org/10.1016/j.jalz.2012.05.2117
Panza F, Lozupone M, Logroscino G, Imbimbo BP (2019) A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat Rev Neurol 15:73–88. https://doi.org/10.1038/s41582-018-0116-6
Ostrowitzki S, Lasser RA, Dorflinger E et al (2017) A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res Ther 9:95. https://doi.org/10.1186/s13195-017-0318-y
Vandenberghe R, Rinne JO, Boada M et al (2016) Bapineuzumab for mild to moderate Alzheimer’s disease in two global, randomized, phase 3 trials. Alzheimers Res Ther 8:18. https://doi.org/10.1186/s13195-016-0189-7
Ostrowitzki S, Bittner T, Sink KM et al (2022) Evaluating the safety and efficacy of crenezumab vs placebo in adults with early Alzheimer disease: two phase 3 randomized placebo-controlled trials. JAMA Neurol 79:1113. https://doi.org/10.1001/jamaneurol.2022.2909
Budd Haeberlein S, Aisen PS, Barkhof F et al (2022) Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. https://doi.org/10.14283/jpad.2022.30
DiStefano MJ, Alexander GC, Polsky D, Anderson GF (2022) Public opinion regarding U.S. Food and Drug Administration approval of aducanumab and potential policy responses: a nationally representative survey. J Am Geriatr Soc 70:1685–1694. https://doi.org/10.1111/jgs.17692
Aisen PS, Cummings J, Doody R et al (2020) The future of anti-amyloid trials. J Prev Alzheimers Dis:1–6. https://doi.org/10.14283/jpad.2020.24
Villain N, Planche V, Levy R (2022) High-clearance anti-amyloid immunotherapies in Alzheimer’s disease. Part 1: meta-analysis and review of efficacy and safety data, and medico-economical aspects. Rev Neurol (Paris) 178:1011–1030. https://doi.org/10.1016/j.neurol.2022.06.012
Sims JR, Zimmer JA, Evans CD et al (2023) Donanemab in early symptomatic alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. https://doi.org/10.1001/jama.2023.13239
Van Dyck CH, Swanson CJ, Aisen P et al (2023) Lecanemab in early Alzheimer’s disease. N Engl J Med 388:9–21. https://doi.org/10.1056/NEJMoa2212948
Smith J, Donohue MC, Gruendl E et al (2023) GRADUATE I AND II: findings of two phase iii randomized placebo-controlled studies assessing the efficacy and safety of subcutaneous gantenerumab in early Alzheimer’s disease (AD) (S26.010). In: Tuesday, April 25. Lippincott Williams & Wilkins, p 4285
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ l4898. https://doi.org/10.1136/bmj.l4898
Higgins J, Thomas J, Chandler J et al (2019) Cochrane handbook for systematic reviews of interventions, 2nd ed. John Wiley & Sons, Chichester (UK)
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ n71. https://doi.org/10.1136/bmj.n71
Cummings JL, Cohen S, Van Dyck CH et al (2018) ABBY: a phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology 90:e1889–e1897. https://doi.org/10.1212/WNL.0000000000005550
Salloway S, Honigberg LA, Cho W et al (2018) Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer’s disease (BLAZE). Alzheimers Res Ther 10:96. https://doi.org/10.1186/s13195-018-0424-5
Mintun MA, Lo AC, Duggan Evans C et al (2021) Donanemab in early Alzheimer’s disease. N Engl J Med 384:1691–1704. https://doi.org/10.1056/NEJMoa2100708
Doody RS, Thomas RG, Farlow M et al (2014) Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 370:311–321. https://doi.org/10.1056/NEJMoa1312889
Honig LS, Vellas B, Woodward M et al (2018) Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 378:321–330. https://doi.org/10.1056/NEJMoa1705971
Salloway S, Sperling R, Fox NC et al (2014) Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370:322–333. https://doi.org/10.1056/NEJMoa1304839
Swanson CJ, Zhang Y, Dhadda S et al (2021) A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther 13:80. https://doi.org/10.1186/s13195-021-00813-8
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Andrews JS, Desai U, Kirson NY et al (2019) Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer’s disease clinical trials. Alzheimers Dement Transl Res Clin Interv 5:354–363. https://doi.org/10.1016/j.trci.2019.06.005
Lansdall CJ, McDougall F, Butler LM et al (2022) Establishing clinically meaningful change on outcome assessments frequently used in trials of mild cognitive impairment due to Alzheimer’s disease. J Prev Alzheimers Dis. https://doi.org/10.14283/jpad.2022.102
Schrag A, Schott JM, Alzheimer’s Disease Neuroimaging Initiative (2012) What is the clinically relevant change on the ADAS-Cog? J Neurol Neurosurg Psychiatry 83:171–173. https://doi.org/10.1136/jnnp-2011-300881
Storandt M, Grant EA, Miller JP, Morris JC (2002) Rates of progression in mild cognitive impairment and early Alzheimer’s disease. Neurology 59:1034–1041. https://doi.org/10.1212/WNL.59.7.1034
Author information
Authors and Affiliations
Contributions
JMD (conception and design, study selection, analysis of data, risk of bias assessment, writing draft, review, and editing); PHCLR (acquisition of data, writing draft, and figures); CSD (acquisition of data, analysis of data, risk of bias assessment, writing draft, review, and editing); NF (analysis of data, writing draft, review, and editing); DDPN (study selection, acquisition of data, writing draft); AM (acquisition of data, risk of bias assessment, writing draft); SB (acquisition of data, writing draft, review, and editing); LT (conception and design, figures, writing draft); AN (analysis of data, review ,and editing); PC (conception and design, study selection, review, and editing).
Corresponding author
Ethics declarations
Ethics approval
This is a systematic review and meta-analysis. No ethical approval was required as no new patient data was collected.
Conflict of interest
Dr. Caramelli declares participation in an advisory board for Roche, the sponsor for CREAD, CREAD2, and SCarlet RoAD. Dr. Caramelli has also developed material for continuous medical education and participation as a speaker in symposia sponsored by Aché, Knight Therapeutics, Roche, and Torrent laboratories. The remainder of the authors have no competing interests to declare. The authors did not receive support from any organization for the submitted work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1727 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dantas, J.M., Mutarelli, A., Navalha, D.D.P. et al. Efficacy of anti-amyloid-ß monoclonal antibody therapy in early Alzheimer’s disease: a systematic review and meta-analysis. Neurol Sci 45, 2461–2469 (2024). https://doi.org/10.1007/s10072-023-07194-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07194-w