Abstract
Purpose
To explore the alterations of whole brain functional network using the degree centrality (DC) analysis in neovascular glaucoma (NVG) and the correlation between DC values and NVG clinical indices.
Materials and methods
Twenty NVG patients and twenty normal controls (NC), closely matched in age, sex, and education, were recruited for this study. All subjects underwent comprehensive ophthalmologic examinations and a resting-state functional magnetic resonance imaging (rs-fMRI) scan. The differences in DC values of brain network between NVG and NC groups were analyzed, and correlation analysis was performed to explore the relationships between DC values and clinical ophthalmological indices in NVG group.
Results
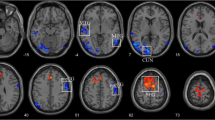
Compared with NC group, significantly decreased DC values were found in the left superior occipital gyrus and left postcentral gyrus, while significantly increased DC values in the right anterior cingulate gyrus and left medial frontal gyrus in NVG group. (All P < 0.05, FDR corrected). In the NVG group, the DC value in left superior occipital gyrus showed significantly positive correlations with retinal nerve fiber layer (RNFL) thickness (R = 0.484, P = 0.031) and mean deviation of visual field (MDVF) (R = 0.678, P = 0.001). Meanwhile, the DC value in the left medial frontal gyrus demonstrated significantly negative correlations with RNFL (R = − 0.544, P = 0.013) and MDVF (R = − 0.481, P = 0.032).
Conclusions
NVG exhibited decreased network degree centrality in visual and sensorimotor brain regions and increased degree centrality in cognitive-emotional processing brain region. Additionally, the DC alterations might be complementary imaging biomarkers to assess disease severity.



Similar content being viewed by others
References
Kwon J, Sung KR (2017) Effect of preoperative intravitreal bevacizumab on the surgical outcome of neovascular glaucoma at different stages. J Ophthalmol 2017:7672485
Rodrigues GB, Abe RY, Zangalli C et al (2016) Neovascular glaucoma: a review. Int J Retina Vitreous 2:26
Yang H, Yu X, Sun X (2018) Neovascular glaucoma: handling in the future. Taiwan J Ophthalmol 8(2):60–66
Senthil S, Dada T, Das T et al (2021) Neovascular glaucoma - a review. Indian J Ophthalmol 69(3):525–534
Călugăru D, Călugăru M (2022) Etiology, pathogenesis, and diagnosis of neovascular glaucoma. Int J Ophthalmol 15(6):1005–1010
Wei HY, Zhang YJ, Zhao SZ (2018) Puerarin regulates neovascular glaucoma through pigment epithelium-derived growth factor-induced NF-κB signaling pathway. Mol Med Rep 17(6):7866–7874
Zhao H, Shi Y, Liang R et al (2021) Voxel-based morphometry reveals altered gray matter volume related to cognitive dysfunctioning in neovascular glaucoma patients. ** 20(4):839–846
Susanna R Jr, De Moraes CG, Cioffi GA, Ritch R (2015) Why do people (still) go blind from glaucoma? Transl Vis Sci Techn 4(2):1
Ramirez AI, de Hoz R, Salobrar-Garcia E et al (2017) The role of microglia in retinal neurodegeneration: Alzheimer's disease, Parkinson, and glaucoma. Front Aging Neurosci 9:214
Ghiso JA, Doudevski I, Ritch R, Rostagno AA (2013) Alzheimer's disease and glaucoma: mechanistic similarities and differences. J Glaucoma 22 Suppl(5):S36–S38
Danesh-Meyer HV, Levin LA (2015) Glaucoma as a neurodegenerative disease. J Neuroophthalmol 35(Suppl 1):S22–S28
Zhang YQ, Peng MY, Wu SN et al (2021) Fractional amplitude of low-frequency fluctuation in patients with neovascular glaucoma: a resting-state functional magnetic resonance imaging study. Quant Imaging Med Surg 11(5):2138–2150
Peng ZY, Liu YX, Li B et al (2021) Altered spontaneous brain activity patterns in patients with neovascular glaucoma using amplitude of low-frequency fluctuations: a functional magnetic resonance imaging study. Brain Behav 11(3):e2018
Yu C, Li CQ, Ge QM et al (2021) Altered resting state functional activity of brain regions in neovascular glaucoma: a resting-state functional magnetic resonance imaging study. Front Neurosci 15:800466
Zuo X, Ehmke R, Mennes M et al (2012) Network centrality in the human functional connectome. Cereb Cortex 22(8):1862–1875
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52(3):1059–1069
Telesford QK, Simpson SL, Burdette JH, Hayasaka S, Laurienti PJ (2011) The brain as a complex system: using network science as a tool for understanding the brain. Brain Connect 1(4):295–308
Tomasi D, Volkow ND (2011) Functional connectivity hubs in the human brain. Neuroimage 57(3):908–917
Wu GR, Stramaglia S, Chen H, Liao W, Marinazzo D (2013) Map** the voxel-wise effective connectome in resting state FMRI. PLOS One 8(9):e73670
Zuo X, **ng X (2014) Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neuroscience & Biobehavioral Reviews 45:100–118
Zhang Q, Shu Y, Li X et al (2019) Resting-state functional magnetic resonance study of primary open-angle glaucoma based on voxelwise brain network degree centrality. Neurosci Lett 712:134500
Cai F, Gao L, Gong H et al (2015) Network centrality of resting-state fMRI in primary angle-closure glaucoma before and after surgery. PLOS One 10(10):e141389
Wang J, Wang X, **a M, Liao X, Evans A, He Y (2015) GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci 9:386
Wang X, Hu W, Wang H et al (2022) Altered structural brain network topology in patients with primary craniocervical dystonia. Front Neurol 13:763305
**a M, Wang J, He Y (2013) BrainNet Viewer: a network visualization tool for human brain connectomics. PLOS One 8(7):e68910
Keitel C, Maess B, Schröger E, Müller MM (2013) Early visual and auditory processing rely on modality-specific attentional resources. Neuroimage 70:240–249
Lalezary M, Medeiros FA, Weinreb RN et al (2006) Baseline optical coherence tomography predicts the development of glaucomatous change in glaucoma suspects. Am J Ophthalmol 142(4):576–582
Iacoboni M, Woods RP, Lenzi GL, Mazziotta JC (1997) Merging of oculomotor and somatomotor space coding in the human right precentral gyrus. Brain: J Neurol 120(9):1635–1645
Rocca MA, Agosta F, Mezzapesa DM et al (2004) A functional MRI study of movement-associated cortical changes in patients with Devic's neuromyelitis optica. Neuroimage 21(3):1061–1068
Silverman SE, Trick GL, Hart WM (1990) Motion perception is abnormal in primary open-angle glaucoma and ocular hypertension. Invest Ophth Vis Sci 31(4):722–729
Wu J, Coffey M, Reidy A, Wormald R (1998) Impaired motion sensitivity as a predictor of subsequent field loss in glaucoma suspects: the Roscommon Glaucoma Study. Brit J Ophthalmol 82(5):534
Delvecchio G, Rossetti MG, Caletti E et al (2019) The neuroanatomy of somatoform disorders: a magnetic resonance imaging study. Psychosomatics 60(3):278–288
Kimmerly DS (2017) A review of human neuroimaging investigations involved with central autonomic regulation of baroreflex-mediated cardiovascular control. Autonomic Neuroscience 207:10–21
Bryden DW, Johnson EE, Tobia SC, Kashtelyan V, Roesch MR (2011) Attention for learning signals in anterior cingulate cortex. J Neurosci 31(50):18266–18274
Blanchard TC, Strait CE, Hayden BY (2015) Ram** ensemble activity in dorsal anterior cingulate neurons during persistent commitment to a decision. J Neurophysiol 114(4):2439–2449
Urien L, **ao Z, Dale J, Bauer EP, Chen Z, Wang J (2018) Rate and temporal coding mechanisms in the anterior cingulate cortex for pain anticipation. Sci Rep-Uk 8(1):1–15
Shinoura N, Yamada R, Tabei Y et al (2013) The right dorsal anterior cingulate cortex may play a role in anxiety disorder and visual function. Neurol Res 35(1):65–70
Ford KA, Goltz NC, Brown MR, Everling S (2005) Neural processes associated with antisaccade task performance investigated with event-related FMRI. J Neurophysiol 94(1):429–440
Schettino A, Loeys T, Delplanque S, Pourtois G (2011) Brain dynamics of upstream perceptual processes leading to visual object recognition: a high density ERP topographic map** study. Neuroimage 55(3):1227–1241
Maunsell JH, Nealey TA, DePriest DD (1990) Magnocellular and parvocellular contributions to responses in the middle temporal visual area (MT) of the macaque monkey. J Neurosci 10(10):3323–3334
Harenski CL, Hamann S (2006) Neural correlates of regulating negative emotions related to moral violations. Neuroimage 30(1):313–324
Seitz RJ, Nickel J, Azari NP (2006) Functional modularity of the medial prefrontal cortex: involvement in human empathy. Neuropsychology 20(6):743–751
Huang X, Li HJ, Peng DC et al (2019) Altered brain network centrality in patients with late monocular blindness: a resting-state fMRI study. Arch Med Sci 15(5):1301–1307
Zhang D, Fan Z, Gao X et al (2018) Illness uncertainty, anxiety and depression in Chinese patients with glaucoma or cataract. Sci Rep 8(1):11671
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81871341 and 82271945).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The protocols of this study were approved by the Local Ethics Committee on Human Research of Eye & ENT Hospital of Fudan University.
Informed Consent
All subjects participated in this study after providing written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Wang, R., Wang, Y. et al. The alterations of brain network degree centrality in patients with neovascular glaucoma: a resting-state fMRI study. Neurol Sci 44, 2915–2922 (2023). https://doi.org/10.1007/s10072-023-06664-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06664-5