Abstract
Introduction
Despite the involvement of subcortical brain structures in the pathogenesis of classic trigeminal neuralgia (CTN), the details of morphological abnormalities of basal ganglia to this disorder are still unknown. This study aimed to investigate potential changes in terms of volume and shape of subcortical regions in patients with CTN.
Methods
Forty-eight patients with CTN and 46 matched healthy subjects were recruited in the study. The whole-brain T1 anatomical data was acquired at a 3.0 Tesla scanner using a fast spoiled gradient recalled sequence (FSPGR). Vertex-wise analysis was applied to detect the alterations of volume and shape in each subcortical region in the patients with CTN compared to healthy controls. The relationships of morphological abnormalities in subcortical structures to the severity of orofacial pain and the affective disturbance in the patient group were examined using the multiple linear regression model.
Results
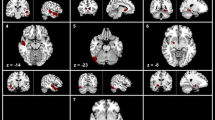
No group difference was found about volumetric measurement in any of the subcortical regions. Vertex-wise analysis revealed areas of significant shape atrophy in bilateral putamen and bilateral pallidum in the patients with CTN compared to healthy controls. Besides, the patient group exhibited shape expansion in the head of the right caudate nucleus compared to healthy subjects. In addition, shape deformation in the head of the right caudate nucleus was positively associated with VAS score in CTN.
Conclusion
The patients with CTN display shape alterations in the specific subregions of basal ganglia, which may contribute to the pathophysiology of this refractory disorder and may be useful for translational medicine.



Similar content being viewed by others
References
Cruccu G, Finnerup NB, Jensen TS, Scholz J, Sindou M, Svensson P et al (2016) Trigeminal neuralgia: New classification and diagnostic grading for practice and research. Neurology 87(2):220–228. https://doi.org/10.1212/wnl.0000000000002840
Cruccu G, Di Stefano G, Truini A (2020) Trigeminal neuralgia. N Engl J Med 383(8):754–762. https://doi.org/10.1056/NEJMra1914484
Bendtsen L, Zakrzewska JM, Heinskou TB, Hodaie M, Leal PRL, Nurmikko T et al (2020) Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol 19(9):784–796. https://doi.org/10.1016/s1474-4422(20)30233-7
Hao Y-b, Zhang W-j, Chen M-j, Chai Y, Zhang W-h, Wei W-b (2020) Sensitivity of magnetic resonance tomographic angiography for detecting the degree of neurovascular compression in trigeminal neuralgia. Neurol Sci 41(10):2947–51. https://doi.org/10.1007/s10072-020-04419-0
Rapisarda A Baroni S Gentili V Moretti G Burattini B Sarlo F et al (2022) The role of biomarkers in drug-resistant trigeminal neuralgia: a prospective study in patients submitted to surgical treatment. Neuro Sci. https://doi.org/10.1007/s10072-022-05971-7
Kakizawa Y, Seguchi T, Kodama K, Ogiwara T, Sasaki T, Goto T et al (2008) Anatomical study of the trigeminal and facial cranial nerves with the aid of 3.0-tesla magnetic resonance imaging. J Neurosurg 108(3):483–90. https://doi.org/10.3171/jns/2008/108/3/0483
Adamczyk M, Bulski T, Sowińska J, Furmanek A, Bekiesińska-Figatowska M (2007) Trigeminal nerve - artery contact in people without trigeminal neuralgia - MR study. Med Sci Monit Int Med J Exp Clin Res 13(Suppl 1):38–43
Ramesh VG, Premkumar G (2009) An anatomical study of the neurovascular relationships at the trigeminal root entry zone. J Clin Neurosci 16(7):934–936. https://doi.org/10.1016/j.jocn.2008.09.011
Obermann M, Rodriguez-Raecke R, Naegel S, Holle D, Mueller D, Yoon MS et al (2013) Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage 74:352–358. https://doi.org/10.1016/j.neuroimage.2013.02.029
Wang Y, Cao D-y, Remeniuk B, Krimmel S, Seminowicz DA, Zhang M (2017) Altered brain structure and function associated with sensory and affective components of classic trigeminal neuralgia. Pain 158(8):1561–70
Wang Y, Yang Q, Cao D, Seminowicz D, Remeniuk B, Gao L et al (2019) Correlation between nerve atrophy, brain grey matter volume and pain severity in patients with primary trigeminal neuralgia. Cephalalgia 39(4):515–525. https://doi.org/10.1177/0333102418793643
Noorani A, Hung PS, Zhang JY, Sohng K, Laperriere N, Moayedi M et al (2022) Pain relief reverses hippocampal abnormalities in trigeminal neuralgia. J Pain 23(1):141–155. https://doi.org/10.1016/j.jpain.2021.07.004
Li M, Yan J, Li S, Wang T, Zhan W, Wen H et al (2017) Reduced volume of gray matter in patients with trigeminal neuralgia. Brain Imaging Behav 11(2):486–492. https://doi.org/10.1007/s11682-016-9529-2
Tsai YH, Yuan R, Patel D, Chandrasekaran S, Weng HH, Yang JT et al (2018) Altered structure and functional connection in patients with classical trigeminal neuralgia. Hum Brain Mapp 39(2):609–621
Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011) A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56(3):907–922. https://doi.org/10.1016/j.neuroimage.2011.02.046
Reckziegel D, Abdullah T, Wu B, Wu B, Huang L, Schnitzer TJ et al (2021) Hippocampus shape deformation: a potential diagnostic biomarker for chronic back pain in women. Pain 162(5):1457–1467. https://doi.org/10.1097/j.pain.0000000000002143
Mao CP, Bai ZL, Zhang XN, Zhang QJ, Zhang L (2016) Abnormal subcortical brain morphology in patients with knee osteoarthritis: a cross-sectional study. Front Aging Neurosci 8:3. https://doi.org/10.3389/fnagi.2016.00003
Xu H Tao Y Zhu P Li D Zhang M Bai G et al (2021) Restoration of aberrant shape of caudate sub-regions associated with cognitive function improvement in mild traumatic brain injury. J Neurotrauma. https://doi.org/10.1089/neu.2021.0426
Xu H, Guo C, Luo F, Sotoodeh R, Zhang M, Wang Y (2019) Subcortical brain abnormalities and clinical relevance in patients with hemifacial spasm. Front Neurol 10:1383. https://doi.org/10.3389/fneur.2019.01383
Yang Q, Xu H, Zhang M, Wang Y, Li D (2020) Volumetric and functional connectivity alterations in patients with chronic cervical spondylotic pain. Neuroradiology 62(8):995–1001. https://doi.org/10.1007/s00234-020-02413-z
Li D Xu H Yang Q Zhang M Wang Y. Cerebral white matter alterations revealed by multiple diffusion metrics in cervical spondylotic patients with pain: a TBSS study. Pain Med. 2021. https://doi.org/10.1093/pm/pnab227
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) Fsl Neuroimage 62(2):782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015
Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A et al (2002) Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 17(1):479–489
Mao CP, Yang HJ (2015) Smaller amygdala volumes in patients with chronic low back pain compared with healthy control individuals. J Pain 16(12):1366–1376. https://doi.org/10.1016/j.jpain.2015.08.012
Chudler EH, Dong WK (1995) The role of the basal ganglia in nociception and pain. Pain 60(1):3–38. https://doi.org/10.1016/0304-3959(94)00172-b
Borsook D, Upadhyay J, Chudler EH, Becerra L (2010) A key role of the basal ganglia in pain and analgesia–insights gained through human functional imaging. Mol Pain 6:27. https://doi.org/10.1186/1744-8069-6-27
Lim M, O’Grady C, Cane D, Goyal A, Lynch M, Beyea S et al (2020) Threat prediction from schemas as a source of bias in pain perception. J Neurosci 40(7):1538–1548. https://doi.org/10.1523/jneurosci.2104-19.2019
Gustin SM, Peck CC, Wilcox SL, Nash PG, Murray GM, Henderson LA (2011) Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J Neurosci 31(16):5956–5964. https://doi.org/10.1523/jneurosci.5980-10.2011
Haber SN, Calzavara R (2009) The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull 78(2):69–74. https://doi.org/10.1016/j.brainresbull.2008.09.013
Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS et al (2011) The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain 134(Pt 7):1987–2004. https://doi.org/10.1093/brain/awr117
Schwab BC, van Wezel RJ, van Gils SA (2017) Sparse pallidal connections shape synchrony in a network model of the basal ganglia. Eur J Neurosci 45(8):1000–1012
Haber SN (2014) The place of dopamine in the cortico-basal ganglia circuit. Neuroscience 282:248–257. https://doi.org/10.1016/j.neuroscience.2014.10.008
Bishop JH, Shpaner M, Kubicki A, Clements S, Watts R, Naylor MR (2018) Structural network differences in chronic muskuloskeletal pain: Beyond fractional anisotropy. Neuroimage 182:441–455. https://doi.org/10.1016/j.neuroimage.2017.12.021
Absinta M, Rocca MA, Colombo B, Falini A, Comi G, Filippi M (2012) Selective decreased grey matter volume of the pain-matrix network in cluster headache. Cephalalgia 32(2):109–115. https://doi.org/10.1177/0333102411431334
Opris I, Lebedev M, Nelson RJ (2011) Motor planning under unpredictable reward: modulations of movement vigor and primate striatum activity. Front Neurosci 5:61. https://doi.org/10.3389/fnins.2011.00061
Grahn JA, Parkinson JA, Owen AM (2008) The cognitive functions of the caudate nucleus. Prog Neurobiol 86(3):141–155. https://doi.org/10.1016/j.pneurobio.2008.09.004
Postuma RB, Dagher A (2006) Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex 16(10):1508–1521. https://doi.org/10.1093/cercor/bhj088
Schmidt-Wilcke T (2008) Variations in brain volume and regional morphology associated with chronic pain. Curr Rheumatol Rep 10(6):467–474. https://doi.org/10.1007/s11926-008-0077-7
Rodgers HM Patton R Yow J Zeczycki TN Kew K Clemens S et al (2021) Morphine resistance in spinal cord injury-related neuropathic pain in rats is associated with alterations in dopamine and dopamine-related metabolomics. J Pain. https://doi.org/10.1016/j.jpain.2021.11.009
Hagelberg N, Martikainen IK, Mansikka H, Hinkka S, Någren K, Hietala J et al (2002) Dopamine D2 receptor binding in the human brain is associated with the response to painful stimulation and pain modulatory capacity. Pain 99(1–2):273–279. https://doi.org/10.1016/s0304-3959(02)00121-5
Fu J, Mu G, Qiu L, Zhao J, Ou C (2020) c-Abl-p38α signaling pathway mediates dopamine neuron loss in trigeminal neuralgia. Mol Pain 16:1744806920930855. https://doi.org/10.1177/1744806920930855
Burkey AR, Carstens E, Jasmin L (1999) Dopamine reuptake inhibition in the rostral agranular insular cortex produces antinociception. J Neurosci 19(10):4169–4179. https://doi.org/10.1523/jneurosci.19-10-04169.1999
Admon R, Holsen LM, Aizley H, Remington A, Whitfield-Gabrieli S, Goldstein JM et al (2015) Striatal hypersensitivity during stress in remitted individuals with recurrent depression. Biol Psychiatry 78(1):67–76. https://doi.org/10.1016/j.biopsych.2014.09.019
Funding
This study was funded by the National Natural Science Foundation of China (82171909), the Key Research and Development Program of Shaanxi (No. 2021SF-091), and the Clinical Research Award of the First Affiliated Hospital of **’an Jiaotong University (No. XJTU1AF-CRF-2020–017).
Author information
Authors and Affiliations
Contributions
H. X. and Y. W. conceived the study, designed the trial, and obtained research funding. H. X. and M. Z. supervised the conduct of the trial and data collection. H. X. provided statistical advice on the study design and analyzed the data; H. X. and Y. W. drafted the manuscript, and all authors contributed substantially to its revision. Y. W. took responsibility for the paper as a whole.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved and consented to by the Ethics Committee of the First Affiliated Hospital of **’an Jiaotong University. Informed consent was obtained from all individual participants according to the Declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, H., Zhang, M. & Wang, Y. Shape deformations of the basal ganglia in patients with classical trigeminal neuralgia: a cross-sectional evaluation. Neurol Sci 43, 5007–5015 (2022). https://doi.org/10.1007/s10072-022-06091-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06091-y