Abstract
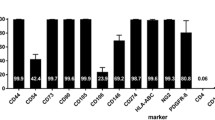
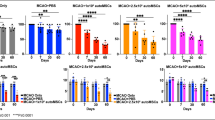
The reorganization of brain structures after intracerebral hemorrhage (ICH) insult is crucial to functional outcome. Although the pattern of neuronal rewiring is well-documented after ischemic stroke, the study of brain plasticity after ICH has been focusing on the enhancement of dendritic complexity. Here we hypothesized that functional restoration after ICH involves brain reorganization which may be favorably modulated by stem cell transplantation. In this study, bone marrow stromal cells (BMSCs) were transplanted into the perilesional sites of collagenaseinduced ICH in adult rats one day after ICH injury. Forelimb functional recovery was monitored with modified limb placing and vibrissae-elicited forelimb placement tests. Anterograde and retrograde tracing were used to assess the reorganization of bilateral forelimb areas of the sensorimotor cortex. We found that in rats transplanted with BMSCs after ICH injury, axonal sprouting occurred in the contralateral caudal forelimb area of the cortex, and was significantly higher than in ICH rat models that received only the vehicle (P < 0.01). The number of positive neurons in the ipsilateral rostral forelimb area of the cortex of the BMSC group was 1.5-to 4.5-fold greater than in the vehicle group (P < 0.05). No difference was found between the BMSC and vehicle groups in hemispheric atrophy or labeled neurons in the ipsilateral caudal forelimb area (P = 0.193). Scores for improved functional behavior in the BMSC group were in accord with the results from histology. Neuronal plasticity of the denervated corticospinal tract at bilateral forelimb areas of the cortex in the collagenase-induced ICH rat models was significantly enhanced by BMSC transplantation. BMSC transplantation may facilitate functional recovery after ICH injury.
Similar content being viewed by others
References
Andres, R.H., Horie, N., Slikker, W., Keren-Gill, H., Zhan, K., Sun, G., Manley, N.C., Pereira, M.P., Sheikh, L.A., McMillan, E.L., et al. (2011). Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 134, 1777–1789.
Auriat, A.M., Wowk, S., and Colbourne, F. (2010). Rehabilitation after intracerebral hemorrhage in rats improves recovery with enhanced dendritic complexity but no effect on cell proliferation. Behav. Brain Res. 214, 42–47.
Barth, T.M., Jones, T.A., and Schallert, T. (1990). Functional subdivisions of the rat somatic sensorimotor cortex. Behav. Brain Res. 39, 73–95.
Borlongan, C.V., Glover, L.E., Tajiri, N., Kaneko, Y., and Freeman, T.B. (2011). The great migration of bone marrow-derived stem cells toward the ischemic brain: Therapeutic implications for stroke and other neurological disorders. Prog. Neurobiol. 95, 213–228.
Bütefisch, C.M., Netz, J., Wessling, M., Seitz, R.J., and Hömberg, V. (2003). Remote changes in cortical excitability after stroke. Brain 126, 470–481.
Candelise, L., Gattinoni, M., Bersano, A., Micieli, G., Sterzi, R., Morabito, A., and PROSIT Study Group (2007). Stroke-unit care for acute stroke patients: an observational follow-up study. Lancet 369, 299–305.
Carmichael, S.T., Archibeque, I., Luke, L., Nolan, T., Momiy, J., and Li, S. (2005). Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp. Neurol. 193, 291–311.
Cohen-Cory, S., and Fraser, S.E. (1995). Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature 378, 192–196.
Conner, J.M., Chiba, A.A., and Tuszynski, M.H. (2005). The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron 46, 173–179.
Daadi, M.M., Davis, A.S., Arac, A., Li, Z., Maag, A.L., Bhatnagar, R., Jiang, K., Sun, G., Wu, J.C., and Steinberg, G.K. (2010). Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke 41, 516–523.
Feng, M., Zhu, H., Zhu, Z., Wei, J., Lu, S., Li, Q., Zhang, N., Li, G., Li, F., Ma, W., et al. (2011). Serial 18F-FDG PET demonstrates benefit of human mesenchymal stem cells in treatment of intracerebral hematoma: a translational study in a primate model. J. Nucl. Med. 52, 90–97.
Ferbert, A., Priori, A., Rothwell, J.C., Day, B.L., Colebath, J.G., and Marsden, C.D. (1992). Interhemispheric inhibition of the human motor cortex. J. Physiol. 453, 525–546.
Gerloff, C., Bushara, K., Sailer, A., Wassermann, E.M., Chen, R., Matsuoka, T., Waldvogel, D., Wittenberg, G.F., Ishii, K., Cohen, L.G., et al. (2006). Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain 129, 791–808.
Grefkes, C., Nowak, D.A., Eickhoff, S.B., Dafotakis, M., Küst, J., Karbe, H., and Fink, G.R. (2008). Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann. Neurol. 63, 236–246.
Horan, P.K., and Slezak, S.E. (1989). Stable cell membrane labelling. Nature 340, 167–168.
Hua, Y., Schallert, T., Keep, R.F., Wu, J., Hoff, J.T., and **, G. (2002). Behavioral tests after intracerebral hemorrhage in the rat. Stroke 33, 2478–2484.
Huang, E.J., and Reichardt, L.F. (2001). Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736.
Jeong, S.W., Chu, K., Jung, K.H., Kim, S.U., Kim, M., and Roh, J.K. (2003). Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke 34, 2258–2263.
**, S.Z., Meng, X.W., Sun, X., Han, M.Z., Liu, B.R., Wang, X.H., and Pei, F.H. (2011). Hepatocyte growth factor promotes liver regeneration induced by transfusion of bone marrow mononuclear cells in a murine acute liver failure model. J. Hepatobiliary Pancreat. Sci. 18, 397–405.
Karp, J.M., and Leng Teo, G.S. (2009). Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4, 206–216.
Kleindorfer, D., Broderick, J., Khoury, J., Flaherty, M., Woo, D., Alwell, K., Moomaw, C.J., Schneider, A., Miller, R., and Kissela, B. (2006). The unchanging incidence and case-fatality of stroke in the 1990s: a population-based study. Stroke 37, 2473–2478.
Lee, S.T., Chu, K., Jung, K.H., Kim, S.J., Kim, D.H., Kang, K.M., Hong, N.H., Kim, J.H., Ban, J.J., Park, H.K., et al. (2008). Antiinflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain 131, 616–629.
Liu, Z., Li, Y., Zhang, Z.G., Cui, X., Cui, Y., Lu, M., Savant-Bhonsale, S., and Chopp, M. (2010). Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. J. Cereb. Blood Flow Metab. 30, 1288–1295.
MacLellan, C.L., Silasi, G., Poon, C.C., Edmundson, C.L., Buist, R., Peeling, J., and Colbourne, F. (2008). Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J. Cereb. Blood Flow Metab. 28, 516–525.
MacLellan, C.L., Silasi, G., Auriat, A.M., and Colbourne, F. (2010). Rodent models of intracerebral hemorrhage. Stroke 41, S95–S98.
Murphy, T.H., and Corbett, D. (2009). Plasticity during stroke recovery: from synapse to behavior. Nat. Rev. Neurosci. 10, 861–872.
Nagai, A., Kim, W.K., Lee, H.J., Jeong, H.S., Kim, K.S., Hong, S.H., Park, I.H., and Kim, S.U. (2007). Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS One 2, e1272.
Nguyen, A.P., Huynh, H.D., Sjovold, S.B., and Colbourne, F. (2008). Progressive brain damage and alterations in dendritic arborization after collagenase-induced intracerebral hemorrhage in rats. Curr. Neurovasc. Res. 5, 171–177.
Otero, L., Zurita, M., Bonilla, C., Aguayo, C., Vela, A., Rico, M.A., and Vaquero, J. (2011). Late transplantation of allogeneic bone marrow stromal cells improves neurologic deficits subsequent to intracerebral hemorrhage. Cytotherapy 13, 562–571.
Otero, L., Zurita, M., Bonilla, C., Aguayo, C., Rico, M.A., Rodríguez, A., and Vaquero, J. (2012). Allogeneic bone marrow stromal cell transplantation after cerebral hemorrhage achieves cell transdifferentiation and modulates endogenous neurogenesis. Cytotherapy 14, 34–44.
Prockop, D.J. (1997). Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276, 71–74.
Ramanathan, D., Conner, J.M., and Tuszynski, M.H. (2006). A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc. Natl. Acad. Sci. USA 103, 11370–11375.
Reitmeir, R., Kilic, E., Reinboth, B.S., Guo, Z., ElAli, A., Zechariah, A., Kilic, U., and Hermann, D.M. (2012). Vascular endothelial growth factor induces contralesional corticobulbar plasticity and functional neurological recovery in the ischemic brain. Acta Neuropathol. 123, 273–284.
Ries, C., Egea, V., Karow, M., Kolb, H., Jochum, M., and Neth, P. (2007). MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood 109, 4055–4063.
Rosenberg, G.A., Mun-Bryce, S., Wesley, M., and Kornfeld, M. (1990). Collagenase-induced intracerebral hemorrhage in rats. Stroke 21, 801–807.
Shimizu, T., Hosaki, A., Hino, T., Sato, M., Komori, T., Hirai, S., and Rossini, P.M. (2002). Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain 125, 1896–1907.
Wang, Y., and Kurata, K. (1998). Quantitative analyses of thalamic and cortical origins of neurons projecting to the rostral and caudal forelimb motor areas in the cerebral cortex of rats. Brain Res. 781, 137–147.
Wieloch, T., and Nikolich, K. (2006). Mechanisms of neural plasticity following brain injury. Curr. Opin. Neurobiol. 16, 258–264.
Wilkins, A., Kemp, K., Ginty, M., Hares, K., Mallam, E., and Scolding, N. (2009). Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 3, 63–70.
**, G., Keep, R.F., and Hoff, J.T. (2006). Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 5, 53–63.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
About this article
Cite this article
Liang, H., Yin, Y., Lin, T. et al. Transplantation of bone marrow stromal cells enhances nerve regeneration of the corticospinal tract and improves recovery of neurological functions in a collagenase-induced rat model of intracerebral hemorrhage. Mol Cells 36, 17–24 (2013). https://doi.org/10.1007/s10059-013-2306-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-013-2306-9