Abstract
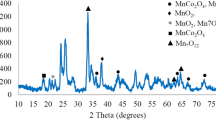
Electrochemical CO2 reduction (CO2RE) using pure metal oxide electrocatalysts such as SnO2, Co3O4, and CuO supported on Vulcan XC72 carbon could be an alternative to overcome the problems caused by high CO2 emission rates. Electrochemically, CO2 can be converted into high-value-added organic compounds such as hydrocarbons and multicarbons. Thus, among the various metal oxides already described in the literature, SnO2 and Co3O4 show good results in catalyzing the CO2 reduction reaction to intermediate products such as formate or methanoate. CuO in turn is capable of reducing CO2 to several products such as methane (CH4), ethylene (C2H4), formate (HCOO−), ethanol (C2H5OH), n-propanol (C3H7OH), and carbon monoxide (CO). Pure Sn, Co, and Cu oxides supported on Vulcan XC72 carbon were synthesized by the co-precipitation method. X-ray diffraction was performed to characterize the crystal structures obtained. SnO2 and SnO2/C had a rutile-type crystalline structure with a tetragonal crystalline system and space group (P42/mnm), while Co3O4 and Co3O4/C had a cubic crystalline system centered on a normal spinel and space group Fd3m, while CuO and CuO/C had a monoclinic structure and a square planar crystalline system with space group C2/C. The materials were characterized by cyclic voltammetry. SnO2 and SnO2/C showed an anodic peak (AI) at −0.81 V vs. Ag/AgCl, which is related to the formation of SnO2, while Co3O4 and Co3O4/C showed an anodic peak (AI) at 0.76 V vs. Ag/AgCl, which is related to the formation of cobalt(IV), and a cathodic peak (CI) at 0.64 V vs. Ag/AgCl, which is related to the reduction of cobalt(IV) to cobalt(III). CuO/C showed anodic peaks at 0.2 V, 0.6 V vs. Ag/AgCl, and cathodic peaks at −0.4 V and −0.8 V vs. Ag/AgCl 3.0 mol L−1 reference electrode. Using linear sweep voltammetry, CuO/C saturated with N2 and saturated with CO2 showed a higher current density compared to the other electrocatalysts, indicating that the H2 evolution reaction was favored, and CO2RE was favored when saturated with CO2. Therefore, this electrocatalyst was selected to study the products formed. The products were studied by nuclear magnetic resonance (NMR) after performing chronoamperometry, showing the potential of this low-cost electrocatalyst for CO2 reduction when formate, ethanol, acetone, acetate, and ethanol were observed at chemical shifts of 8.5, 3.2, 2.2, 1.9, and 1.2 ppm.








Similar content being viewed by others
Data availability
Data will be made available on request.
References
Podder J, Patra BR, Pattnaik F et al (2023) A review of carbon capture and valorization technologies. Energies 16:2589. https://doi.org/10.3390/en16062589
Intergovernmental Panel on Climate Change (2023) Climate change 2021 – the physical science basis: working group I contribution to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge. https://www.academia.edu/67940264/Mudan%C3%A7as_clim%C3%A1ticas_na_agenda_global_O_que_aprendemos_com_as_Confer%C3%AAncias_das_Partes_COP_e_o_que_est%C3%A1_em_jogo_na_COP_26. Accessed 2 Jan 2024
Jardim WF (2001) A evolução da atmosfera terrestre. http://qnesc.sbq.org.br/online/cadernos/01/evolucao.pdf. Accessed 2 Jan 2024
de Tilio Neto P (2010) Ecopolítica das mudanças climáticas: o IPCC e o ecologismo dos pobres. https://doi.org/10.11606/T.8.2008.tde-09102008-175152
Ma T, Fan Q, Li X et al (2019) Graphene-based materials for electrochemical CO2 reduction. J CO2 Util 30:168–182. https://doi.org/10.1016/j.jcou.2019.02.001
Benson EE, Kubiak CP, Sathrum AJ, Smieja JM (2009) Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem Soc Rev 38:89–99. https://doi.org/10.1039/B804323J
Ishiki NA, Lima FHB, Ticianelli EA (2022) Redução eletroquímica de CO2: refazendo nossas pegadas de carbono. Química Nov na Esc 45(2):109–116. https://doi.org/10.21577/0104-8899.20160323
da Silva GTST, Lopes OF, Dias EH et al (2021) Redução de CO2 em hidrocarbonetos e oxigenados: fundamentos, estratégias e desafios. Quím Nova 44:963–981. https://doi.org/10.21577/0100-4042.20170745
Khezri B, Fisher AC, Pumera M (2017) CO2 reduction: the quest for electrocatalytic materials. J Mater Chem A 5:8230–8246. https://doi.org/10.1039/C6TA09875D
Zhan Z, Zhao L (2010) Electrochemical reduction of CO2 in solid oxide electrolysis cells. J Power Sources 195:7250–7254. https://doi.org/10.1016/j.jpowsour.2010.05.037
Cardoso ES (2012) Síntese e caracterização de eletrocatalisadores Pt/C, PtAu/C e PtAuBi/C pelo método da redução via feixe de elétrons para oxidação direta de metanol e etanol. Mestrado em Tecnologia Nuclear - Materiais, Universidade de São Paulo. https://www.teses.usp.br/teses/disponiveis/85/85134/tde-07112012-074656/publico/2012CardosoSintese.pdf. Accessed 2 Jan 2024
Hori Y (2008) Electrochemical CO2 reduction on metal electrodes. Modern aspects of electrochemistry. Springer New York, New York, NY, pp 89–189. https://doi.org/10.1007/978-0-387-49489-0_3
Raciti D, Wang C (2018) Recent advances in CO2 reduction electrocatalysis on copper. ACS Energy Lett 3:1545–1556. https://doi.org/10.1021/acsenergylett.8b00553
Gao S, Lin Y, Jiao X et al (2016) Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 529:68–71. https://doi.org/10.1038/nature16455
Li J, Jiao J, Zhang H et al (2020) Two-dimensional SnO2 nanosheets for efficient carbon dioxide electroreduction to formate. ACS Sustainable Chem Eng 8:4975–4982. https://doi.org/10.1021/acssuschemeng.0c01070
Yue Y, Zou X, Shi Y et al (2023) A low crystallinity CuO-SnO2/C catalyst for efficient electrocatalytic reduction of CO2. J Electroanal Chem 928:117089. https://doi.org/10.1016/j.jelechem.2022.117089
Gao S, Jiao X, Sun Z et al (2016) Ultrathin Co3O4 layers realizing optimized CO2 electroreduction to formate. Angew Chem 128:708–712. https://doi.org/10.1002/ange.201509800
Carmo MD (2008) Preparação, caracterização e avaliação de carbono funcionalizado para aplicações em células a combustível tipo PEM. Doutorado em Tecnologia Nuclear - Materiais, Universidade de São Paulo. https://doi.org/10.11606/T.85.2008.tde-10102011-144752
Redín I, Gisela G (2016) Functionalization and characterization of carbon black Vulcan XC-72R for applications on inhibition-based enzymatic biosensors. https://repositorio.ufscar.br/handle/ufscar/7893. Accessed 2 Jan 2024
Cheng Y, Hou P, Pan H et al (2020) Selective electrocatalytic reduction of carbon dioxide to oxalate by lead-tin oxides with low overpotential. Appl Catal B 272:118954. https://doi.org/10.1016/j.apcatb.2020.118954
Rangel WM (2014) Síntese de nanopartículas de óxido de cobre (II) pelo método de coprecipitação. Dissertação, Universidade Federal de Santa Catarina. https://repositorio.ufsc.br/xmlui/handle/123456789/132428. Accessed 2 Jan 2024
Debataraja A, Zulhendri DW, Yuliarto B et al (2017) Investigation of nanostructured SnO2 synthesized with polyol technique for CO gas sensor applications. Procedia Engineering 170:60–64. https://doi.org/10.1016/j.proeng.2017.03.011
Wan N, Lu X, Wang Y et al (2016) Improved Li storage performance in SnO2 nanocrystals by a synergetic do**. Sci Rep 6:18978. https://doi.org/10.1038/srep18978
Louardi A, Rmili A, Ouachtari F et al (2011) Characterization of cobalt oxide thin films prepared by a facile spray pyrolysis technique using perfume atomizer. J Alloy Compd 509:9183–9189. https://doi.org/10.1016/j.jallcom.2011.06.106
Prabaharan DDM, Sadaiyandi K, Mahendran M, Sagadevan S (2017) Precipitation method and characterization of cobalt oxide nanoparticles. Appl Phys A 123:264. https://doi.org/10.1007/s00339-017-0786-8
de Souza F, M, Paniz FP, Pedron T, et al (2019) A high-throughput analytical tool for quantification of 15 metallic nanoparticles supported on carbon black. Heliyon 5:e01308. https://doi.org/10.1016/j.heliyon.2019.e01308
Rathod D, Vijay M, Islam N et al (2009) Design of an “all solid-state” supercapacitor based on phosphoric acid doped polybenzimidazole (PBI) electrolyte. J Appl Electrochem 39:1097–1103. https://doi.org/10.1007/s10800-008-9764-3
Barman M, Mosali VSS, Bond AM et al (2023) Multi-products (C1 and C2) formation from electrochemical reduction of carbon dioxide catalyzed by oxide-derived coppers prepared using varied synthesis conditions. Electrocatalysis 14:511–521. https://doi.org/10.1007/s12678-023-00814-1
Hu Q, Huo Q, Qi S et al (2023) Unconventional synthesis of hierarchically twinned copper as efficient electrocatalyst for nitrate–ammonia conversion. Adv Mater n/a:2311375. https://doi.org/10.1002/adma.202311375
Merino MCG, Palermo M, Belda R et al (2012) Combustion synthesis of Co3O4 nanoparticles: fuel ratio effect on the physical properties of the resulting powders. Procedia Materials Science 1:588–593. https://doi.org/10.1016/j.mspro.2012.06.079
Gu F, Li C, Hu Y, Zhang L (2007) Synthesis and optical characterization of Co3O4 nanocrystals. J Cryst Growth 304:369–373. https://doi.org/10.1016/j.jcrysgro.2007.03.040
Senthilkumar V, Vickraman P, Jayachandran M, Sanjeeviraja C (2010) Synthesis and characterization of SnO2 nanopowder prepared by precipitation method. J Dispersion Sci Technol 31:1178–1181. https://doi.org/10.1080/01932690903223856
Rangel WM, Boca Santa RAA, Riella HG (2020) A facile method for synthesis of nanostructured copper (II) oxide by coprecipitation. J Market Res 9:994–1004. https://doi.org/10.1016/j.jmrt.2019.11.039
Puppin LG, da Silva LF, Carmo M et al (2021) Effect of the oxidation state and morphology of SnOx-based electrocatalysts on the CO2 reduction reaction. J Mater Res 36:4240–4248. https://doi.org/10.1557/s43578-021-00250-1
Lobo AO, Martin AA, Antunes EF et al (2005) Caracterização de materiais carbonosos por espectroscopia RAMAN. Rev Bras Apl Vácuo 24:98–103. https://doi.org/10.17563/rbav.v24i2.99
Ticianelli EA, Gonzalez ER (1998) Eletroquímica: princípios e aplicações. Edusp, São Paulo
González S, Pérez M, Barrera M et al (1998) Mechanism of copper passivation in aqueous sodium carbonate−bicarbonate solution derived from combined X-ray photoelectron spectroscopic and electrochemical data. J Phys Chem B 102:5483–5489. https://doi.org/10.1021/jp981069k
Pérez-Rodríguez S, Pastor E, Lázaro MJ (2017) Noble metal-free catalysts supported on carbon for CO2 electrochemical reduction. J CO2 Util 18:41–52. https://doi.org/10.1016/j.jcou.2017.01.010
Landers AT, Fields M, Torelli DA et al (2018) The predominance of hydrogen evolution on transition metal sulfides and phosphides under CO2 reduction conditions: an experimental and theoretical study. ACS Energy Lett 3:1450–1457. https://doi.org/10.1021/acsenergylett.8b00237
Carmo M, Dos Santos AR, Poco JGR, Linardi M (2007) Physical and electrochemical evaluation of commercial carbon black as electrocatalysts supports for DMFC applications. J Power Sources 173:860–866. https://doi.org/10.1016/j.jpowsour.2007.08.032
Lázaro MJ, Calvillo L, Celorrio V et al (2011) Study and application of Vulcan XC-72 in low-temperature fuel cells, pp 41–68. https://www.researchgate.net/profile/Veronica-Celorrio/publication/259442510_Study_and_application_of_Vulcan_XC-72_in_low_temperature_fuel_cells/links/0046353709edbb2b19000000/Study-and-application-of-Vulcan-XC-72-in-low-temperature-fuel-cells.pdf. Accessed 2 Jan 2024
Barcelos MM, de Vasconcellos MLS, Ribeiro J (2024) Recent progress in electrochemical CO2 reduction at different electrocatalyst materials. Processes 12:303. https://doi.org/10.3390/pr12020303
Darayen J, Chailapakul O, Praserthdam P, Panpranot J, Tungasmita DN, Boonyongmaneerat Y (2021) Porous electrodeposited Cu as a potential electrode for electrochemical reduction reactions of CO2. Appl Sci 11:11104. https://doi.org/10.3390/app112311104
Atta-ur-Rahman, Choudhary MI (2015) Applications of NMR spectroscopy. Elsevier
Acknowledgements
We are grateful to the Carlos Alberto Redins Cellular Ultrastructure Laboratory (LUCCAR/CCS) at UFES, for conducting SEM, EDS, and TEM analyses. A special acknowledgment goes to the Carbonaceous and Ceramic Materials Laboratory (LMC/CCE) at UFES for their assistance with XRD analyses. We are also thankful to the Physicochemical and Microbiological Characterization Laboratory (LACAR) – SECTI for the Raman analysis. In addition, we would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES) and Espírito Santo Research Support Foundation (FAPES) funding agencies for their financial support, which played a crucial role in making this research possible.
Funding
FAPES, CAPES, and CNPq.
Author information
Authors and Affiliations
Contributions
Robson R. Garcia: conceptualization, investigation, methodology, writing—original draft; Gabriel F. S. dos Santos: formal analysis, visualization, writing—review and editing; Alvaro Cunha Neto: formal analysis; Josimar Ribeiro: writing—review and editing, project administration, supervision, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garcia, R.R., dos Santos, G.F.S., Neto, A.C. et al. Synthesis of electrocatalysts based on MxOy and MxOy/C (M = Sn, Cu, and Co) with potential for application in CO2 reduction. J Solid State Electrochem (2024). https://doi.org/10.1007/s10008-024-05903-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10008-024-05903-9