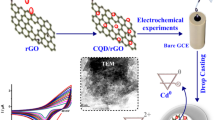
Abstract
Thiram is a pesticide derived from sulfur that has a highly toxic and biologically active chemical molecule due to its ability to chelate polyvalent cations, being the second most used fungicide in agriculture. Due to its toxic effects, which include liver damage, neurotoxicity, infertility problems and bone and cartilaginous malformations, among others, the detection of this contaminant in water is essential. This research work describes the development of a new glassy carbon electrode modified with electrochemically reduced graphene oxide and zinc oxide nanosheets (GCE-ErGO-ZnO) to detect the presence of the pesticide thiram by electrochemical means. The GCE-ErGO-ZnO electrode was examined morphologically and chemically and analyzed by cyclic voltammetry in the presence of thiram. Electrochemical characterization demonstrated that GCE-ErGO-ZnO presents the highest electrocatalytic activity for thiram oxidation using ZnO nanosheets. Thiram was successfully identified by the square wave voltammetry method in Britton-Robinson buffer 0.1 mol·L−1, at pH = 5.0. The developed electrochemical sensor allows the quantification of thiram in the linear range 0.09–0.96 μg·mL−1, with a LOD of 1.3 ng·mL−1 and LOQ of 4.3 ng·mL−1, significantly lower than the maximum Brazilian and global concentration levels, demonstrating that this method has significant potential to be used in monitoring this pesticide in watercourses.
Graphical Abstract







Similar content being viewed by others
References
Manahan SE (2013) Fundamentals of environmental and toxicological chemistry, 4th edn. CRC Press, New York, USA
Singh NS, Sharma R, Parween T, Patanjali PK (2018) Pesticide contamination and human health risk factor. In: Oves M, Khan MZ, Ismail IMI (eds) Modern Age Environmental Problems and their Remediation. 1st ed, Springer Cham, pp 49–68
Syafrudin M, Kristanti RA, Yuniarto A et al (2021) Pesticides in drinking water — a review. Int J Environ Res Public Health 18:468. https://doi.org/10.3390/ijerph18020468
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications, 2nd edn. John Wiley & Sons, New York
Peres F, Moreira JC, Dubois GS (2003) Is it poison or is it medicine?: Pesticides, health and environment: an introduction to the topic. Pesticides, health and environment: an introduction to the topic, 20th edn. Editora FIOCRUZ, Rio de Janeiro, pp 21–41
Boger B, Tonin FS, Guillermo P et al (2015) Pharmaceutical micropollutants in brazilian aqueous samples: A systematic review. Ciência e Nat 37:725–739
Steter JR, Kossuga MH, Motheo AJ (2016) Mechanistic proposal for the electrochemical and sonoelectrochemical oxidation of thiram on a boron-doped diamond anode. Ultrason Sonochem 28:21–30. https://doi.org/10.1016/j.ultsonch.2015.06.022
Nováková K, Navrátil T, Dytrtová JJ, Chýlková J (2013) The use of copper solid amalgam electrodes for determination of the pesticide thiram. J Solid State Electrochem 17:1517–1528. https://doi.org/10.1007/s10008-013-2035-1
Maximiano EM, de Lima F, Cardoso CAL, Arruda GJ (2018) Modification of carbon paste electrodes with recrystallized zeolite for simultaneous quantification of thiram and carbendazim in food samples and an agricultural formulation. Electrochim Acta 259:66–76. https://doi.org/10.1016/j.electacta.2017.10.162
Parham H, Pourreza N, Marahel F (2015) Determination of thiram using gold nanoparticles and resonance rayleigh scattering method. Talanta 141:143–149. https://doi.org/10.1016/j.talanta.2015.03.061
Nabi F, Shahzad M, Liu J et al (2016) Hsp90 inhibitor celastrol reinstates growth plate angiogenesis in thiram-induced tibial dyschondroplasia. Avian Pathol 45:187–193. https://doi.org/10.1080/03079457.2016.1141170
Matthiaschk G (1973) Über den Einfluß von L-Cystein auf die Teratogenese durch Thiram (TMTD) bei NMRI-Mäusen. Arch Toxicol 30:251–262. https://doi.org/10.1007/bf02426049
Fishbein L (1976) Environmental health aspects of fungicides. I Dithiocarbamates J Toxicol Environ Health 1:713–735. https://doi.org/10.1080/15287397609529371
Commission E (2018) Commission Implementing Regulation (EU) 2018/1500 of 9 October 2018, concerning the non-renewal of approval of the active substance thiram, and prohibiting the use and sale of seeds treated with plant protection products containing thiram. Off J Eur Union 254:2018–2020. https://doi.org/10.2903/j.efsa.2017.4700
Ibáñez D, Izquierdo-Bote D, González-García MB et al (2021) Development of a new screen-printed transducer for the electrochemical detection of thiram. Chemosensors 9(11):303. https://doi.org/10.3390/chemosensors9110303
Anvisa (2019) Resolution of the collegiate board - RDC No. 320, of November 28th, 2019 - Provides for the maintenance of the active ingredient Thiram in pesticide products in the country, as well as determining measures to mitigate health risks and changes in the resul. Brazil
Crnogorac G, Schwack W (2009) Residue analysis of dithiocarbamate fungicides. TrAC - Trends Anal Chem 28:40–50. https://doi.org/10.1016/j.trac.2008.10.008
Huang Y, Zhou Q, **e G (2013) Development of sensitive determination method for fungicides from environmental water samples with Titanate nanotube array micro-solid phase extraction prior to high performance liquid chromatography. Chemosphere 90:338–343. https://doi.org/10.1016/j.chemosphere.2012.07.024
Kim KG, Park DW, Kang GR et al (2016) Simultaneous determination of plant growth regulator and pesticides in bean sprouts by liquid chromatography-tandem mass spectrometry. Food Chem 208:239–244. https://doi.org/10.1016/j.foodchem.2016.04.002
Rastegarzadeh S, Abdali S (2013) Colorimetric determination of thiram based on formation of gold nanoparticles using ascorbic acid. Talanta 104:22–26. https://doi.org/10.1016/j.talanta.2012.11.023
Lanças FM (2004) Validation of chromatographic analysis methods, 1st ed. RiMa, São Carlos
Hercegová A, Dömötörová M, Matisová E (2007) Sample preparation methods in the analysis of pesticide residues in baby food with subsequent chromatographic determination. J Chromatogr A 1153:54–73. https://doi.org/10.1016/j.chroma.2007.01.008
Alves CC, Coelho MKL, Pereira AC (2020) Electrochemical Sensors Based on Different Materials for Pesticides Determination. Rev Virtual Quim 12:1599–1625. https://doi.org/10.21577/1984-6835.20200124
Moses PR, Wler L, Murray RW (1975) Chemically Modified Tin Oxide Electrode. Anal Chem 47:1882–1886. https://doi.org/10.1021/ac60362a043
Santana PCA, Lima JBS, Santana TBS et al (2019) Semiconductor nanocrystals-reduced graphene composites for the electrochemical detection of carbendazim. J Braz Chem Soc 30:1302–1308. https://doi.org/10.21577/0103-5053.20190026
Pereira AC, Santos ADS, Kubota LT (2002) Trends in modification of amperometric electrodes for electroanalytical applications. Quim Nova 25:1012–1021. https://doi.org/10.1590/s0100-40422002000600019
de Souza M, FB, (1997) Chemically modified electrodes applied to electroanalysis: a brief approach. Quim Nova 20:191–195. https://doi.org/10.1590/S0100-40421997000200011
Singh V, Joung D, Zhai L et al (2011) Graphene based materials: Past, present and future. Prog Mater Sci 56:1178–1271. https://doi.org/10.1016/j.pmatsci.2011.03.003
Novoselov KS, Geim AK, Morozov SV et al (2004) Electric Field Effect in Atomically Thin Carbon Films Supplementary. Science 349(6245):290–295. https://doi.org/10.1126/science.aab1343
Geim AK, Novoselov KS (2007) The rise of graphene PROGRESS. Nat Mater 6:183–191
Roy S, Soin N, Bajpai R et al (2011) Graphene oxide for electrochemical sensing applications. J Mater Chem 21:14725–14731. https://doi.org/10.1039/c1jm12028j
Assis KLDSC, Archanjo BS, Achete CA, D’Elia E (2020) A New Sensor Based on Reduced Graphene Oxide/Au Nanoparticles for Glycerol Detection. Mater Res 23(2). https://doi.org/10.1590/1980-5373-mr-2019-0513
Katz E, Willner I, Wang J (2004) Electroanalytical and bioelectroanalytical systems based on metal and semiconductor nanoparticles. Electroanalysis 16(1–2):19–44. https://doi.org/10.1002/elan.200302930
Luo X, Morrin A, Killard AJ, Smyth MR (2006) Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 18:319–326. https://doi.org/10.1002/elan.200503415
Wang F, Hu S (2009) Electrochemical sensors based on metal and semiconductor nanoparticles. Microchim Acta 165:1–22. https://doi.org/10.1007/s00604-009-0136-4
Wei A, Pan L, Huang W (2011) Recent progress in the ZnO nanostructure-based sensors. Mater Sci Eng B Solid-State Mater Adv Technol 176:1409–1421. https://doi.org/10.1016/j.mseb.2011.09.005
Zhao Z, Lei W, Zhang X et al (2010) ZnO-based amperometric enzyme biosensors Sensors 10:1216–1231. https://doi.org/10.3390/s100201216
Rohilla D, Chaudhary S, Umar A (2021) An Overview of Advanced Nanomaterials for Sensor Applications. Eng Sci 16:47–70. https://doi.org/10.30919/es8d552
Que M, Lin C, Sun J et al (2021) Progress in zno nanosensors Sensors 21:1–22. https://doi.org/10.3390/s21165502
Li F, Ni B, Zheng Y et al (2021) A simple and efficient voltammetric sensor for dopamine determination based on ZnO nanorods/electro-reduced graphene oxide composite. Surfaces and Interfaces 26:101375. https://doi.org/10.1016/j.surfin.2021.101375
Sulciute A, Nishimura K, Gilshtein E et al (2021) ZnO Nanostructures Application in Electrochemistry: Influence of Morphology. J Phys Chem C 125:1472–1482. https://doi.org/10.1021/acs.jpcc.0c08459
Shetti NP, Malode SJ, Ilager D et al (2019) A Novel Electrochemical Sensor for Detection of Molinate Using ZnO Nanoparticles Loaded Carbon Electrode. Electroanalysis 31:1040–1049. https://doi.org/10.1002/elan.201800775
Wang C, Song Q, Liu X, Zhu X (2020) Development of electrochemical sensor based on graphene oxide electrode modified by silver-doped ZNO nanorods for detection of carbamate pesticide in food. Int J Electrochem Sci 15(6):5623–5631. https://doi.org/10.20964/2020.06.74
Hummers WS, Offeman RE (1958) Preparation of Graphitic Oxide. J Am Chem Soc 80:1339. https://doi.org/10.1021/ja01539a017
Wojnarowicz J, Chudoba T, Koltsov I et al (2018) Size control mechanism of ZnO nanoparticles obtained in microwave solvothermal synthesis. Nanotechnology 29(6):065601. https://doi.org/10.1088/1361-6528/aaa0ef
Castro KLS, Oliveira SM, Curti RV et al (2018) Electrochemical response of glassy carbon electrodes modified using graphene sheets of different sizes. Int J Electrochem Sci 13:71–87. https://doi.org/10.20964/2018.01.02
De Camargo MNL, Santhiago M, Maroneze CM et al (2016) Tuning the electrochemical reduction of graphene oxide: Structural correlations towards the electrooxidation of nicotinamide adenine dinucleotide hydride. Electrochim Acta 197:194–199. https://doi.org/10.1016/j.electacta.2015.09.022
Cochran WG (1941) The distribution of the largest of a set of estimated variances as a fraction of their total. Ann Eugen 11:47–52. https://doi.org/10.1111/j.1469-1809.1941.tb02271.x
Eurachem (2014) Eurachem guide: the fitness for purpose of analytical methods - a laboratory guide to method validation and related topics. 2nd ed. Eurachem, United Kingdom
Massart DL, Vandeginste BGM, Buydens LMC et al (1997) Handbook of Chemometrics and Qualimetrics: Part A By Data Handling in Science and Technology, 1st edn. Elsevier, Amsterdam
Ribani M, Grespan Bottoli CB, Collins CH et al (2004) Validation in chromatographic and electrophoretic methods. Quim Nova 27:771–780. https://doi.org/10.1590/S0100-40422004000500017
Maximiano EM, Cardoso CAL, Arruda GJ (2020) Simultaneous electroanalytical determination of thiram and carbendazim in samples of fresh fruit juices in the presence of surfactants. Food Anal Methods 13:119–130. https://doi.org/10.1007/s12161-019-01550-3
Wojnarowicz J, Chudoba T, Lojkowski W (2020) A review of microwave synthesis of zinc oxide nanomaterials: Reactants, process parameters and morphoslogies. Nanomaterials 10(6):1086. https://doi.org/10.3390/nano10061086
Zanatta C dos S (2009) Preparation and characterization of nanostructured zinc oxide. Master’s dissertation. Universidade Estadual Paulista (UNESP)
Wagner CD, Gale LH, Raymond RH (1979) Two-dimensional chemical state plots: a standardized data set for use in identifying chemical states by x-ray photoelectron spectroscopy. Anal Chem 51:466–482. https://doi.org/10.1021/ac50040a005
Prakash T, Jayaprakash R, Neri G, Kumar S (2013) Synthesis of ZnO nanostructures by microwave irradiation using albumen as a template. J Nanoparticles 2013:1–8. https://doi.org/10.1155/2013/274894
Kong XR, Duan Y, Peng P et al (2007) A novel route to prepare ZnO nanotubes by using microwave irradiation method. Chem Lett 36:428–429. https://doi.org/10.1246/cl.2007.428
Kumar SS, Venkateswarlu P, Rao VR, Rao GN (2013) Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int Nano Lett 3:389–394. https://doi.org/10.1557/proc-452-389
Lee S, Jeong S, Kim D et al (2008) ZnO nanoparticles with controlled shapes and sizes prepared using a simple polyol synthesis. Superlattices Microstruct 43:330–339. https://doi.org/10.1016/j.spmi.2008.01.004
Bodke MR, Purushotham Y, Dole BN (2018) Comparative study on zinc oxide nanocrystals synthesized by two precipitation methods. Ceramica 64:91–96. https://doi.org/10.1590/0366-69132018643692207
Al-Gaashani R, Najjar A, Zakaria Y et al (2019) XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram Int 45:14439–14448. https://doi.org/10.1016/j.ceramint.2019.04.165
Engstrom RC (1982) Electrochemical pretreatment of glassy carbon electrodes. Anal Chem 54:2310–2314. https://doi.org/10.1021/ac00250a038
Engstrom RC, Strasser VA (1984) Characterization of electrochemically pretreated glassy carbon electrodes. Anal Chem 56:136–141. https://doi.org/10.1021/ac00266a005
Kepley LJ, Bard AJ (1988) Ellipsometric, electrochemical, and elemental characterization of the surface phase produced on glassy carbon electrodes by electrochemical activation. Anal Chem 60:1459–1467. https://doi.org/10.1021/ac00165a022
McCreery RL (2008) Advanced carbon electrode materials for molecular electrochemistry. Chem Rev 108:2646–2687. https://doi.org/10.1021/cr068076m
Noel M, Anantharaman PN (1986) Voltammetric studies on glassy carbon electrodes I: Electrochemical behaviour of glassy carbon electrodes in H2SO4, Na2SO4 and NaOH media. Surf Coatings Technol 28:161–179. https://doi.org/10.1016/0257-8972(86)90055-1
Walcarius A (1998) Analytical applications of silica-modified electrodes - a comprehensive review. Electroanalysis 10:1217–1235. https://doi.org/10.1002/(sici)1521-4109(199812)10:18%3c1217::aid-elan1217%3e3.0.co;2-x
Craievich AF (1976) On the structure of glassy carbon. Mater Res Bull 11:1249–1255. https://doi.org/10.1016/0025-5408(76)90029-5
Downard AJ (2000) Electrochemically assisted covalent modification of carbon electrodes. Electroanalysis 12:1085–1096. https://doi.org/10.1002/1521-4109(200010)12:14%3c1085::AID-ELAN1085%3e3.0.CO;2-A
Botas C, Álvarez P, Blanco C et al (2012) The effect of the parent graphite on the structure of graphene oxide. Carbon N Y 50:275–282. https://doi.org/10.1016/j.carbon.2011.08.045
Wu F, Lu Y, Shao G et al (2012) Preparation of polyacrylonitrile/graphene oxide by in situ polymerization. Polym Int 61:1394–1399. https://doi.org/10.1002/pi.4221
Acik M, Chabal YJ (2012) A review on reducing graphene oxide for band gap engineering. J Mater Sci Res 2:101–112. https://doi.org/10.5539/jmsr.v2n1p101
Chua CK, Pumera M (2014) Chemical reduction of graphene oxide: a synthetic chemistry viewpoint. Chem Soc Rev 43:291–312. https://doi.org/10.1039/C3CS60303B
Agarwal S, Jangir LK, Rathore KS et al (2019) Morphology-dependent structural and optical properties of ZnO nanostructures. Appl Phys A Mater Sci Process 125:1–7. https://doi.org/10.1007/s00339-019-2852-x
Shi LE, Li ZH, Zheng W et al (2014) Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: A review. Food Addit Contam - Part A 31:173–186. https://doi.org/10.1080/19440049.2013.865147
Umar A, Hahn YB (2006) ZnO nanosheet networks and hexagonal nanodiscs grown on silicon substrate: Growth mechanism and structural and optical properties. Nanotechnology 17:2174–2180. https://doi.org/10.1088/0957-4484/17/9/016
Al-Gaashani R, Radiman S, Daud AR et al (2013) XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram Int 39:2283–2292. https://doi.org/10.1016/j.ceramint.2012.08.075
Xue J, Luo Z, Li P et al (2014) A residue-free green synergistic antifungal nanotechnology for pesticide thiram by ZnO nanoparticles. Sci Rep 4:1–9. https://doi.org/10.1038/srep05408
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39:228–240. https://doi.org/10.1039/B917103G
Wong A, da Dias ACMS, Dutra RAF, Sotomayor MDPT (2013) Development and application of a screen-printed electrode modified with MWCNT for the electrocatalytic detection of thiram. Curr Top Electrochem 17:87–94
Bandeira NS, Abreu CD, De OR (2019) Detection of Thiram fungicide through electrochemical activation of printed carbon electrodes. Acta Iguazu 8:1–12
Wei X, Liu C, Li Z et al (2022) Fabrication of a label-free electrochemical cell-based biosensor for toxicity assessment of thiram. Chemosphere 307:135960. https://doi.org/10.1016/j.chemosphere.2022.135960
Ragam PN, Mathew B (2020) Unmodified silver nanoparticles for dual detection of dithiocarbamate fungicide and rapid degradation of water pollutants. Int J Environ Sci Technol 17:1739–1752. https://doi.org/10.1007/s13762-019-02454-9
Rocchitta G, Spanu A, Babudieri S et al (2016) Enzyme biosensors for biomedical applications: strategies for safeguarding analytical performances in biological fluids. Sensors 16:780. https://doi.org/10.3390/s16060780
Rasheed T, Bilal M, Nabeel F et al (2019) Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ Int 122:52–66. https://doi.org/10.1016/j.envint.2018.11.038
Anvisa (2021) Pesticide registration analysis program: information about analysis of thiram concentration in food. In: Brazil. https://www.gov.br/anvisa/pt-br/assuntos/agrotoxicos/programa-de-analise-de-residuos-emalimentos. Accessed 12 Apr 2021
Association of Official Analytical Chemists (AOAC) (2023) Appendix D - guidelines for collaborative study procedures to validate characteristics of a method of analysis. In: Latimer GW (ed) Official methods of analysis of AOAC International. Oxford University PressNew York, Washington, pp 694–704
ICH (2005) Validation of analytical procedures: Text and methodology, in Q2(R1), 4th edn. ICH harmonised tripartite guideline, London
Mnif W, Hassine AIH, Bouaziz A et al (2011) Effect of endocrine disruptor pesticides: A review. Int J Environ Res Public Health 8:2265–2303. https://doi.org/10.3390/ijerph8062265
Acknowledgements
To the postgraduate studies program of Brazil’s National Institute of Metrology, Quality and Technology (PPGM-INMETRO). To Rede Fluminense para Pesquisa e Desenvolvimento de Nanomateriais e Nanobiossistemas. To Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). To Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Financiadora de Estudos e Projetos (FINEP). To Microscopy Lab (LABMI) of Brazil’s National Institute of Metrology, Quality and Technology (INMETRO). To Oleksii Kuznetsov for the XRD analysis. To Joyce Rodrigues de Araujo for XPS analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pedro, N., Assis, K., Archanjo, B. et al. A new sensor based on ZnO nanosheets and reduced graphene oxide to electrochemical determination of thiram fungicide. J Solid State Electrochem (2024). https://doi.org/10.1007/s10008-024-05857-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10008-024-05857-y