Abstract
Objectives
This cross-sectional invitro research aimed to compare and contrast the macroscopic and microscopic, mechanical and biochemical features of leukocyte-rich platelet-rich fibrin, advanced platelet-rich fibrin, and injectable platelet-rich fibrin.
Materials and methods
In all, 150 samples were taken from males aged 18 to 25 with good systemic health (n = 50 each for i-PRF, A-PRF, and L-PRF). The samples were assessed for clot length, clot width, membrane length and width. Microscopic parameters assessed were the distribution of cells and fibrin structure. Mechanical tests were performed for tensile strength using a universal testing machine and growth factor analysis was performed for platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and transforming growth factor (TGF)- \(\beta\) on Days 1, 3 and 7 using commercially available ELISA kits. The osteogenic potential was analyzed in a culture of human periodontal ligament cells for 21 days using cell viability assay, alkaline phosphatase formation and alizarin red staining for mineralization.
Results
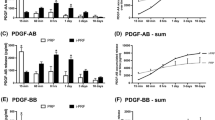
L-PRF demonstrates statistically superior clot length, width, weight, membrane length, width and weight in comparison to A-PRF (p < 0.05). L-PRF demonstrates a denser fibrin structure in comparison to A-PRF and i-PRF (p < 0.05). The cells in L-PRF are most commonly situated in the proximal of the clot where as they are distributed in the proximal and middle aspect for A-PRF(p < 0.05). A-PRF demonstrates the highest tensile strength followed by L-PRF (p < 0.05). When growth factor release was evaluated, A-PRF showed noticeably increased release of all growth factors, namely PDGF-BB, TGF-ß, and VEGF, in comparison to i-PRF and L-PRF (p < 0.05). On days 7 and 14, the cell viability of human periodontal ligament cells in co-culture with A-PRF was statistically substantially greater than that of L-PRF and i-PRF (p < 0.05). Alkaline phosphatase levels were statistically substantially higher in A-PRF, followed by i-PRF and L-PRF on days 14 and 21 (p < 0.05). After 21 days of culture, A-PRF treated cultures had much more Alizarin Red staining than L-PRF and i-PRF cultures did (p < 0.05).
Conclusion
It was determined that although L-PRF exhibits greater size and weight in comparison to A-PRF and i-PRF, A-PRF has superior mechanical properties, increased growth factor releases of TGF-b, PDGF-BB, and VEGF as well as superior cell viability, alkaline phosphatase production, and mineralization on human periodontal ligament cells.
Clinical relevance
Based on these findings, A-PRF can be recommended for improved delivery of growth factors and osteogenesis whereas L-PRF is better-suited for applications relying on the size of membrane.






Similar content being viewed by others
Data availability
Data supporting the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions, but can be shared upon request. Requests for access to the data should be directed to srucha2k@gmail.com.
References
Golebiewska EM, Poole AW (2015) Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev 29:153–162. https://doi.org/10.1016/j.blre.2014.10.003
Mancuso ME, Santagostino E (2017) Platelets: much more than bricks in a breached wall. Br J Haematol 178:209–219. https://doi.org/10.1111/bjh.14653
Nurden AT (2011) Platelets, inflammation and tissue regeneration. Thromb Haemost 105(Suppl 1):S13-33. https://doi.org/10.1160/THS10-11-0720
Everts P, Onishi K, Jayaram P, Lana JF, Mautner K (2020) Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci 21:7794. https://doi.org/10.3390/ijms21207794
Marx RE (2001) Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent 10:225–228
Choukroun J, Adda F, Schoeffler C, Vervelle A (2001) Une opportunité en paro-implantologie: Le PRF. Implantodontie 42:55–62. https://doi.org/10.1051/mbcb/2013074
Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J et al (2014) Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol 40:679–689. https://doi.org/10.1563/aaid-joi-D-14-00138
Mourão C, Valiense H, Melo E, Mourão N, Maia M (2015) Obtention of injectable platelets rich-fibrin (i-PRF) and its polymerization with bone graft: technical note. Rev Col Bras Cir 42:421–423. https://doi.org/10.1590/0100-69912015006013
Choukroun J, Ghanaati S (2018) Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: The first introduction to the low-speed centrifugation concept. Eur J Trauma Emerg Surg 44:87–95. https://doi.org/10.1007/s00068-017-0767-9
Pinto NR, Pereda A, Jiménez P, Corso MD, Kang B, Wang HL et al (2014) The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors and fibrin architecture of a Leukocyte- and Platelet-Rich Fibrin (L-PRF) clot and membrane. Part 2: Macroscopic, photonic microscopy and scanning electron microscopy analysis of 4 kinds of L-PRF clots and membranes. Poseido 2:141–154
Ehrenfest D, Corso M, Kang B, Lanata N, Quiryen M, Wang H et al (2014) The impact of centrifuge characteristics and centrifugation protocols on the cells, growth factors and fibrin architecture of a leukocyte-and platelet rich fibrin (L-PRF) clot and membrane. Part 3: Comparison of the growth factors content and slow release between the original L-PRF and the modified A-PRF (Advanced Platelet-Rich Fibrin) membranes. Poseido 2:155–166
Yajamanya SR, Chatterjee A, Babu CN, Karunanithi D (2016) Fibrin network pattern changes of platelet-rich fibrin in young versus old age group of individuals: A cell block cytology study. J Indian Soc Periodontol 20:151–156. https://doi.org/10.4103/0972-124X.176390
Khorshidi H, Raoofi S, Bagheri R, Banihashemi H (2016) Comparison of the mechanical properties of early Leukocyte- and Platelet-Rich Fibrin versus PRGF/Endoret membranes. Int J Dent: 1849207. https://doi.org/10.1155/2016/1849207
Chang YC, Zhao JH (2011) Effects of platelet-rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Aust Dent J 56:365–371. https://doi.org/10.1111/j.1834-7819.2011.01362.x
Masuki H, Okudera T, Watanebe T, Suzuki M, Nishiyama K, Okudera H, Nakata K, Uematsu K, Su CY, Kawase T (2016) Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int J Implant Dent 2(1):19. https://doi.org/10.1186/s40729-016-0052-4
Miron RJ, Pinto NR, Quirynen M, Ghanaati S (2019) Standardization of relative centrifugal forces in studies related to platelet-rich fibrin. J Periodontol 90:817–820. https://doi.org/10.1002/JPER.18-0553
Dohan Ehrenfest DM, Pinto NR, Pereda A, Jiménez P, Corso MD, Kang BS et al (2018) The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 29:171–184. https://doi.org/10.1080/09537104.2017.1293812
Sam G, Vadakkekuttical RJ, Amol NV (2015) In vitro evaluation of mechanical properties of platelet-rich fibrin membrane and scanning electron microscopic examination of its surface characteristics. J Indian Soc Periodontol 19:32–36. https://doi.org/10.4103/0972-124X.145821
Mrozik K, Gronthos S, Shi S, Bartold PM (2010) A method to isolate, purify, and characterize human periodontal ligament stem cells. Methods Mol Biol 666:269–284. https://doi.org/10.1007/978-1-60761-820-1_17
Miron RJ, Dham A, Dham U, Zhang Y, Pikos MA, Sculean A (2019) The effect of age, gender, and time between blood draw and start of centrifugation on the size outcomes of platelet-rich fibrin (PRF) membranes. Clin Oral Investig 35:2179–2185. https://doi.org/10.1007/s00784-018-2673-x
Bai MY, Chuang MH, Lin MF, Tang SL, Wong CC, Chan WP (2018) Relationships of age and sex with cytokine content and distribution in human platelet fibrin gels. Sci Rep 8:10642. https://doi.org/10.1038/s41598-018-28376-z
Mamajiwala AS, Sethi KS, Raut CP, Karde PA, Mangle NM (2020) Impact of different platelet-rich fibrin (PRF) procurement methods on the platelet count, antimicrobial efficacy, and fibrin network pattern in different age groups: an in vitro study. Clin Oral Investig 24:1663–1675. https://doi.org/10.1007/s00784-019-03022-8
Das N, Amaranath BJJ (2022) Qualitative analysis of modified advanced-platelet-rich fibrin buffy coat among diabetic patients and tobacco smokers with chronic periodontitis: A cell block cytology study. Contemp Clin Dent 13:173–182. https://doi.org/10.4103/ccd.ccd_1018_20
Srirangarajan S, Sindhu V, Prabhu S, Rao RJ, Rudresh V (2021) Does cigarette smoking induce changes in biologic and mechanical properties of platelet-rich fibrin membranes? Int J Periodontics Restorative Dent 41:e213-21. https://doi.org/10.11607/prd.4573
Gupta S, Jain A, Gupta M, Gupta J, Kansal S, Bhansali A et al (2022) Influence of periodontitis and diabetes on structure and cytokine content of platelet-rich fibrin. Oral Dis. https://doi.org/10.1111/odi.14275
Nakahara M (1978) Effect of antibiotics on platelet thromboplastic function and thrombin activity. J Med 9:433–443
Genua MI, Giraldez J, Rocha E, Monge A (1980) Effects of antibiotics on platelet functions in human plasma in vitro and dog plasma in vivo. J Pharm Sci 1980(69):1282–1284. https://doi.org/10.1002/jps.2600691113
Kariyazono H, Nakamura K, Shinkawa T, Moriyama Y, Toyohira H, Taira A et al (1997) Inhibitory effects of antibiotics on platelet aggregation in vitro. Hum Exp Toxicol 16:662–666. https://doi.org/10.1177/096032719701601106
Das N, Janardhana Amaranath BJ (2022) Quantitative evaluation of modified advanced platelet-rich fibrin buffy coat among diabetic patients and tobacco smokers with chronic periodontitis. J Indian Soc Periodontol 26:24–31. https://doi.org/10.4103/jisp.jisp_498_20
Chatterjee A, Debnath K, Ali MM, Babu C, Gowda PL (2017) Comparative histologic evaluation of titanium platelet-rich fibrin and platelet-rich fibrin in hypertensive and smoker participants: A cell cytology study. J Indian Soc Periodontol 21:195–200. https://doi.org/10.4103/jisp.jisp_137_17
Goel A, Windsor LJ, Gregory RL, Blanchard SB, Hamada Y (2021) Effects of platelet-rich fibrin on human gingival and periodontal ligament fibroblast proliferation from chronic periodontitis versus periodontally healthy subjects. Clin Exp Dent Res 7:436–442. https://doi.org/10.1002/cre2.370
Chang J, Blanchard SB, Windsor LJ, Gregory RL, Hamada Y (2020) Levels of growth factors from platelet-rich fibrin from chronic periodontitis versus periodontally healthy subjects: A pilot study. Clin Oral Investig 24:823–832. https://doi.org/10.1007/s00784-019-02944-7
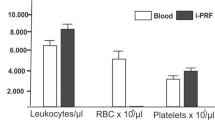
Sonika S, Nivetha R, Esther Nalini H, Arun Kumar Prasad P, Renuka Devi R (2021) Influence of RBC count and haemoglobin concentration on platelet rich fibrin network among different age groups and gender- A cross sectional study. Int J Recent Sci Res 12:41777–80. https://doi.org/10.24327/ijrsr.2021.1205.5961
Crisci A (2019) The L-PRF Membrane (fibrin rich in platelets and leukocytes) and its derivatives (A-PRF, I-PRF) are useful as a source of stem cells in regenerative wound therapy: Experimental work on the horse. Regen Med Ther: 37–45. https://doi.org/10.36959/654/392
Ravi S, Santhanakrishnan M (2020) Mechanical, chemical, structural analysis and comparative release of PDGF-AA from L-PRF, A-PRF and T-PRF - an in vitro study. Biomater Res 24:16. https://doi.org/10.1186/s40824-020-00193-4
Simões-Pedro M, Tróia PMBPS, Dos Santos NBM, Completo AMG, Castilho RM, de Oliveira Fernandes GV (2022) Tensile strength essay comparing three different platelet-rich fibrin membranes (L-PRF, A-PRF, and A-PRF+): A mechanical and structural in vitro evaluation. Polymers (Basel) 14:1392. https://doi.org/10.3390/polym14071392
Kaigler D, Avila G, Wisner-Lynch L, Nevins ML, Nevins M, Rasperini G et al (2011) Platelet-derived growth factor applications in periodontal and peri-implant bone regeneration. Expert Opin Biol Ther 11:375–385. https://doi.org/10.1517/14712598.2011.554814
Ripamonti U, Ferretti C, Teare J, Blann L (2009) Transforming growth factor-beta isoforms and the induction of bone formation: implications for reconstructive craniofacial surgery. J Craniofac Surg 20:1544–1555. https://doi.org/10.1097/SCS.0b013e3181b09ca6
Smith PC, Martínez C, Cáceres M, Martínez J (2015) Research on growth factors in periodontology. Periodontol 2000(67):234–250. https://doi.org/10.1111/prd.12068
Shibuya M (2011) Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2:1097–1105. https://doi.org/10.1177/1947601911423031
Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B et al (2016) Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Invest 20:2353–2360. https://doi.org/10.1007/s00784-016-1719-1
Pitzurra L, Jansen IDC, de Vries TJ, Hoogenkamp MA, Loos BG (2020) Effects of L-PRF and A-PRF+ on periodontal fibroblasts in in vitro wound healing experiments. J Periodontal Res 55:287–295. https://doi.org/10.1111/jre.12714
Dohan Ehrenfest DM, Diss A, Odin G, Doglioli P, Hippolyte MP, Charrier JB (2009) In vitro effects of Choukroun’s PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:341–352. https://doi.org/10.1016/j.joen.2010.07.004
Huang FM, Yang SF, Zhao JH, Chang YC (2010) Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J Endod 36:1628–1712
Wang X, Zhang Y, Choukroun J, Ghanaati S, Miron RJ (2017) Behavior of gingival fibroblasts on titanium implant surfaces in combination with either injectable-PRF or PRP. Int J Mol Sci 18:331. https://doi.org/10.3390/ijms18020331
Thanasrisuebwong P, Surarit R, Bencharit S, Ruangsawasdi N (2019) Influence of fractionation methods on physical and biological properties of injectable platelet-rich fibrin: An exploratory study. Int J Mol Sci 20:1657. https://doi.org/10.3390/ijms20071657
Zheng S, Zhang X, Zhao Q, Chai J, Zhang Y (2020) Liquid platelet-rich fibrin promotes the regenerative potential of human periodontal ligament cells. Oral Dis 26:1755–1763. https://doi.org/10.1111/odi.13501
El Bagdadi K, Kubesch A, Yu X, Al-Maawi S, Orlowska A, Dias A (2019) Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: A proof of concept of LSCC (low speed centrifugation concept). Eur J Trauma Emerg Surg 45:467–479. https://doi.org/10.1007/s00068-017-0785-7
Miron RJ, Chai J, Zhang P, Wang Y, Mourão CFAB, Sculean A et al (2020) A novel method for harvesting concentrated platelet-rich fibrin (C-PRF) with a 10-fold increase in platelet and leukocyte yields. Clin Oral Investig 24:2819–2828. https://doi.org/10.1007/s00784-019-03147-w
Fujioka-Kobayashi M, Katagiri H, Kono M, Schaller B, Zhang Y, Sculean A et al (2020) Improved growth factor delivery and cellular activity using concentrated platelet-rich fibrin (C-PRF) when compared with traditional injectable (i-PRF) protocols. Clin Oral Investig 24:4373–4383. https://doi.org/10.1007/s00784-020-03303-7
Acknowledgements
Dr. K G Bhat, Maratha Mandal Dental College, Belgaum, Karnataka India for carrying out ELISA and Cell culture analysis.
Author information
Authors and Affiliations
Contributions
Shah R: contributed to Conceptualization, Methodology,, Investigation, Resources, Data Collection, Statistics, Writing – Original Draft Preparation, Writing – Review & Editing, Visualization
MG Triveni: contributed to Conceptualization, Methodology, Data Collection, Writing – Review & Editing, Visualization, Approval of final draft
Thomas R: contributed to Conceptualization, Methodology, Data Collection, Statistics, Writing – Review & Editing, Visualization
AB Tarun Kumar: contributed to Conceptualization, Methodology, Data Collection, Writing – Review & Editing, Visualization
Corresponding author
Ethics declarations
Ethical approval
Approved by institutional ethical committee.
Consent to participate/publication
Written consent was obtained from all the participants.
Competing interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, R., M. G., T., Thomas, R. et al. Advanced platelet rich fibrin demonstrates improved osteogenic induction potential in human periodontal ligament cells, growth factor production and mechanical properties as compared to leukocyte and platelet fibrin and injectable platelet rich fibrin. Oral Maxillofac Surg 28, 413–424 (2024). https://doi.org/10.1007/s10006-023-01160-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-023-01160-8