Abstract
Context
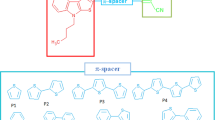
In the present work, the influence of aromatic ring substitution on a series of small-donor organic molecules (A, B, C, D, and E) with isoxazole cores was investigated for photovoltaic applications in organic solar cells. Frontier molecular orbital analysis, chemical reactivity descriptors, dipole moment, and population analysis showed that all the organic materials have intramolecular charge transfer abilities capable of donating electrons to the acceptor material (PCBM). The required photovoltaic parameters such as Voc, FF, Jsc, LHE, and other associated optoelectronic parameters are reported. The results demonstrate that aromatic ring substitution influences charge transfer and power conversion efficiencies of solar cells. That is, an increase in the aromatic character of a material increases its charge transfer, and as a result, its photovoltaic properties are increased. Additionally, all the investigated derivatives are good charge transporters with suitable electron reorganization energies, which are beneficial for minimizing energy loss. Hence, these organic derivatives with isoxazole backbones are promising materials and may provide fresh insights into the design of new materials for organic solar cell applications.
Method
All calculations were performed using DFT and the ORCA 4.1.0 program package as the main tool for geometry optimization and frequency calculations. The Avogadro 1.2.1 visualization tool was used to prepare all input files executed by ORCA 4.1.0. The BP86, B3LYP, and wB97M series of functionals coupled with the def2/TZVP basis set were employed for geometry optimization. All energy-related calculations were carried out using the M06-2x functional. Multiwfn version 3.7 was used for aromaticity and population analysis. Excited state and UV-visible spectra were simulated using the TD-DFT method at the CAM-B3LYP-D3, wB97X-D3, and PBE0-D3 coupled with the ma-def2-TZVP basis set. Moreover, solvent effects were incorporated using the SMD scheme as incorporated in the ORCA software. Lastly, the RIJCOSX approximations were used to speed up calculations while maintaining accuracy.








Similar content being viewed by others
Data availability
Data is provided within the manuscript as well as Supplementary information files.
References
Dasari M, Balaraman RP, Kohli P (2018) Photovoltaics and nanotechnology as alternative energy. Environ Chem Sustain World 1:211–241. https://doi.org/10.1007/978-3-319-76090-2_7
De Wild J, Meijerink A, Rath JK, Van S, Schropp R (2011) Upconverter solar cells: materials and applications. Energy Environ Sci 4:4835–4848. https://doi.org/10.1039/C1EE01659H
Dutta A, Bouri E, Rothovius T, Uddin GS (2023) Climate risk and green investments: new evidence. Energy 265:1–13. https://doi.org/10.1016/j.energy.2022.126376
Tahir KA, Zamorano M, García JO (2023) Scientific map** of optimisation applied to microgrids integrated with renewable energy systems. Int J Electr Power Energy Syst 145:1–18. https://doi.org/10.1016/j.ijepes.2022.108698
Solangi K, Islam M, Saidur R, Rahim N, Fayaz H (2011) A review on global solar energy policy. Renew Sust Energy Rev 15:2149–2163. https://doi.org/10.1016/j.rser.2011.01.007
Greatzel M (2009) Recent advances in sensitized mesoscopic solar cells. Acc Chem Res 42:1788–1798. https://doi.org/10.1021/ar900141y
Meng L, Zhang Y, Wan X, Li C, Zhang X, Wang Y, Ke X, **ao Z, Ding L, **a R, Yip H, Cao Y, Chen Y (2018) Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 361:1094–1098. https://doi.org/10.1126/science.aat2612
Fowzia SA, Sobhi MG, Hoda AA (2021) Optical investigations and photoactive solar energy applications of new synthesized Schiff base liquid crystal derivatives. Sci Rep 11:15046. https://doi.org/10.1038/2Fs41598-021-97264-w
Zhang M, Zhu L, Zhou G, Haol T, Qiu C, Zhao Z, Hu Q, Larson BW, Zhu H, Ma Z, Tang Z, Feng W, Zhang Y, Russell TP, Liu F (2021) Single-layered organic photovoltaics with double cascading charge transport pathways:18% efficiencies. Nat Commun 12:309–319
Irfan A, Mahmood A (2018) Designing of efficient acceptors for organic solar cells: molecular modelling at DFT level. J Clust Sci 30:75–82
Chen H, Hou J, Zhang S, Liang Y, Yang G, Lu** YY, Wu Y, Li G (2009) Polymer solar cells with enhanced open-circuit voltage and efficiency. Nat Photon 3:649–653
Hong L, Yao H, Wu Z, Cui Y, Zhang T, Xu Y, Yu R, Liao Q, Gao B, **an K, Young Woo H, Ge Z, Hou J (2019) Eco-compatible solvent-processed organic photovoltaic cells with over 16% efficiency. Adv Mater 31:e1903441. https://doi.org/10.1002/adma.201903441
Liu S, Yuan J, Deng W, Luo M, **e Y, Liang Q, Zou Y, He Z, Wu H, Cao Y (2020) High-efficiency organic solar cells with low non-radiative recombination loss and low energetic disorder. Nat Photon 14:300–305. https://doi.org/10.1038/s41566-019-0573-5
Lakhwani G, Rao A, Friend RH (2014) Bimolecular recombination in organic photovoltaics. Annu Rev Phys Chem 65:557–581. https://doi.org/10.1146/annurev-physchem-040513-103615
Shi Y, Chang Y, Lu K, Chen Z, Zhang J, Yan Y, Qiu D, Liu Y, Adil MA, Ma W, Hao X, Zhu L, Wei Z (2022) Small reorganization energy acceptors enable low energy losses in non-fullerene organic solar cells. Nat Commun 13:3256–3263. https://doi.org/10.1038/s41467-022-30927-y
Dennler G, Scharber MC, Brabec C (2009) Polymer-fullerene bulk-heterojunction solar cells. Adv Mater 21:1323–1338. https://doi.org/10.1002/adma.200801283
Yassar A, Miozzo L, Gironda R, Horowitz G (2013) Rod-coil and all-conjugated block copolymers for photovoltaic applications. Prog Polym Sci 38:791–844. https://doi.org/10.1016/j.progpolymsci.2012.10.001
Yi Y, Coropceanu V, Bredas JL (2009) Exciton-dissociation and charge-recombination processes in pentacene/C60 solar cells: theoretical Insight into the impact of interface geometry. J Am Chem Soc 131:15777–15783. https://doi.org/10.1021/ja905975w
Mayukh M, Jung IH, He F, Yu L (2012) Incremental optimization in donor polymers for bulk heterojunction organic solar cells exhibiting high performance. J Polym Sci B Polym Phys 50:1057–1070. https://doi.org/10.1002/polb.23102
Sun Y, Welch YG, Leong WL, Takacs CJ, Bazan GC, Heeger AJ (2012) Solution -processed small-molecule solar cells with 6.7% efficiency. Nat Mater 19:44–48
Roncali J, Leriche P, Blanchard P (2014) Molecular materials for organic photovoltaics: small is beautiful. Adv Mater 26:3821–3838. https://doi.org/10.1002/adma.201305999
Ghanem T, Vincendeau T, Simón MP, Habibi AH, Abidi S (2021) Synthesis of push-pull triarylamine dyes containing 5,6difluoro-2,1,3-benzothiadiazole units by direct arylation and evaluation as active material for organic photovoltaics. Adv Mater 2:1–10. https://doi.org/10.1039/D1MA00798J
Kudrjasova J, Van Landeghem M, Vangerven T, Kesters J, Heintges GHL, Cardinaletti I, Lenaerts R, Penxten H, Adriaensens P, Lutsen L, Vanderzande D, Manca J, Goovaerts E, Maes W (2017) Designing Small molecule organic solar cells with high open-circuit voltage. ChemistrySelect 2:1253–1261. https://doi.org/10.1002/slct.201601915
Morita T, Yugandar S, Fuse S, Nakamura H (2018) Recent progresses in the synthesis of functionalized isoxazoles. Tetrahedron Lett 59:1159–1171. https://doi.org/10.1016/j.tetlet.2018.02.020
Guguloth V, Paidakula S, Vadde R (2020) One-pot regioselective synthesis of some novel isoxazole-phenothiazine hybrids and their antibacterial activity. Russ J Gen Chem 90:470–475. https://doi.org/10.1134/S1070363220030214
Kumar RN, Jitender D, Ravikumar N, Swaroop DK, Debanjan B, Bharath G, Narsaiah B, Jain SN, Rao AG Therapeutic potential of heterocyclic pyrimidine scaffolds. Bioorg Med Chem Lett 26:2927. https://doi.org/10.1186/s13065-018-0406-5
Trivedi J, Parveen A, Rozy F, Mitra A, Bal C, Mitra D, Sharon A (2019) Discovery of 2-isoxazol-3-yl-acetamide analogues as heat shock protein 90 (HSP90) inhibitors with significant anti-HIV activity. Eur J Med Chem 183:111699. https://doi.org/10.1016/j.ejmech.2019.111699
Naidu KM, Srinivasarao S, Agnieszka N, Ewa AK, Kumar MMK, Chandra Sekhar KVG (2016) Seeking potent anti-tubercular agents : design, synthesis, anti-tubercular activity and docking study of various ((triazoles/indole)-piperazin-1-yl/1,4-diazepan-1-yl)benzo[d]isoxazole derivatives. Bioorg Med Chem Lett 26:2245–2250. https://doi.org/10.1016/j.bmcl.2016.03.059
Abdelall EKA (2020) Synthesis and biological evaluations of novel isoxazoles and furoxan derivative as anti-inflammatory agents. Bioorg Chem 94:10344. https://doi.org/10.1016/j.bioorg.2019.103441
Sysak A, Obmińska-Mrukowicz B (2017) Isoxazole ring as a useful scaffold in a search for new therapeutic agents. Eur J Med Chem 137(137):292–309. https://doi.org/10.1016/j.ejmech.2017.06.002
Zhua J, Moa J, Lina H-Z, Chenb Y, Suna H-P (2018) The recent progress of isoxazole in medicinal chemistry. Bioorg Med Chem 26:3065–3075
Irfan A, Al-Sehemi AG, Chaudhry AR, Muhammad S (2016) A first principle study of the linear and nonlinear optical properties of isoxazole derivatives. J Theor Comput Chem 15:210–222. https://doi.org/10.1142/S0219633616500607
Reshak AH, Sikander A (2015) Thermoelectric and optoelectronic properties of a heterocyclic isoxazolone nucleus compound. Mater Sci Semicond Process 30:197–207. https://doi.org/10.1016/j.mssp.2014.10.005
Holzer B, Stöger B, Kautny P, Reider G, Hametner C, Fröhlicha J, Lumpi D (2018) A novel selenoalkenyl-isoxazole based donor-acceptor nonlinear optical material. CrystEngComm 20:12–16. https://doi.org/10.1039/c7ce01925d
Kumar V, Koud**a S, Verma P, Chetti P (2023) Optoelectronic design and charge transport properties of Benzodifuran (BDF) isomers for organic electronic devices: DFT/TD-DFT insights. Spectrochim Acta A Mol Biomol Spectrosc 290. https://doi.org/10.1016/j.saa.2022.122266
Koud**a S, Kumar V, Atohoun GYS, Gbenou JD, Chetti P (2023) Impact of organic dye-photosensitizer on TiO2 nanocrystalline surface for high performance organic electronic devices: A computational insight. J. Photochem. Photobiol. A: Chem 442:114772
Heteroatom-bridged heterofluorenes: a theoretical study on molecular structures and optoelectronic properties. Phys Chem Chem Phys 6:3675–3682. https://doi.org/10.1039/C9CP06458C
Shakru R, Subhashini NJP, Sathish KK, Shivaraj S (2010) Synthesis, characterisation and antimicrobial studies on cobalt (II), nickel (II), copper (II) and zinc (II) complexes of N, O, S donor Schiff bases. J Chem Phar Res 2:38–46
Prashanthi Y, Shiva R (2009) Synthesis and characterization of transition metal complexes with N, O; N, N and S, N-donor Schifff base ligands. J Sci Res 2:114–126
Perdew JP (1986) Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B 34:8822–8824. https://doi.org/10.1103/PhysRevB.33.8822
Becke AD (1993) Density-functional thermochemistry III. The role of exact exchange. J Chem Phys 98:5648. https://doi.org/10.1063/1.464913
Lee CT, Yang WT, Parr RG (1988) Development of the colle-salvetti correlation energy formula into a functional of the electron-density. Phys Rev B Condens Matter Mater Phys 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Neese F (2009) Prediction of molecular properties and molecular spectroscopy with density functional theory: from fundamental theory to exchange-coupling. Coord Chem Rev 253:526–563
Kumar V, Tripathi A, Koud**a S, Chetti P (2023) Benzodithiophene (BDT) and benzodiselenophene (BDSe) isomers’ charge transport properties for organic optoelectronic devices. J Sulphur Chem 44:462–478. https://doi.org/10.1080/17415993.2023.2173009
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4:17. https://doi.org/10.1186/1758-2946-4-17
Neese F (2012) The ORCA program system. Comput Mol Sci 2:73–78. https://doi.org/10.1002/wcms.81
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functional. Theor Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Neese F (2003) An improvement of the resolution of the identity approximation for the calculation of the coulomb matrix. J Comput Chem 24:1740–1747. https://doi.org/10.1002/jcc.10318
Neese F, Wennmohs F, Hansen A, Becker U (2009) Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree–Fock exchange. Chem Phys 356:98–109. https://doi.org/10.1016/j.chemphys.2008.10.036
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Bird CW (1985) A new aromaticity index and its application to five-membered ring heterocycles. Tetrahedron 41:1409–1414. https://doi.org/10.1016/S0040-4020(01)96543-3
Poater J, Fradera X, Duran M, Solà M (2003) The delocalization index as an electronic aromaticity criterion: application to a series of planar polycyclic aromatic hydrocarbons. Chem Eur J 9:400–406. https://doi.org/10.1002/chem.200390041
Etabti H, Fitri A, Benjelloun AT, Benzakour M, Mohammed Mcharf M (2021) Efficient tuning of benzocarbazole based small donor molecules with D-π-A-π-D configuration for high-effciency solar cells via π-bridge manipulation: A DFT/ TD-DFT study. Comput Theor Chem 1208:113580. https://doi.org/10.1016/j.comptc.2021.113580
Alongamo LIC, Tasheh NS, Nkungli KN, Bine KF, Ghogomu NJ (2022) Structural, electronic, and charge transport properties of new materials based on 2-(5-mercapto-1,3,4-oxadiazol-2-yl) phenol for organic solar cells and light emitting diodes by DFT and TD-DFT. J Chem 2022:1–15. https://doi.org/10.1155/2022/1802826
Raftani M, Abram T, Bennani N, Bouachrine M (2020) Theoretical study of new conjugated compounds with a low band gap for bulk heterojunction solar cells: DFT and TD-DFT study. Result Chem 2:1–12. https://doi.org/10.1016/j.rechem.2020.100040
Escudero D, Laurent AD, Denis Jacquemin D (2017) Time-dependent density functional theory: a tool to explore excited states handbook of computational chemistry, pp 927–1957. https://doi.org/10.1007/978-3-319-27282-5_43
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378. https://doi.org/10.1021/jp810292n
Wu Z, Fan B, Xue F, Adachi C, Ouyang J (2010) Organic molecules based on dithienyl-2,1,3-benzothiadiazole as new donor materials for solution-processed organic photovoltaic cells. Sol Energy Mater Sol Cells 94:2230–2237
Mhlbacher D, Scharber M, Morana M, Zhu Z, Waller D, Gaudiana R, Brabec C (2006) High photovoltaic performance of a low-bandgap polymer. Adv Mat 18:2884–2288. https://doi.org/10.1002/ADMA.200600160
Ji X, Wang T, Fu Q, Liu D, Wu Z, Zhang M, Woo HY, Liu Y (2023) Deciphering the effects of molecular dipole moments on the photovoltaic performance of organic solar cells. Macromol Rapid Commun. https://doi.org/10.1002/marc.202300213
Zhang ZL, Zou LY, Ren AM, Liu YF, Feng JK, Sun CC (2013) Theoretical studies on the structures and optical properties of star-shaped triazatruxene/heterofluorene co-polymers. Dye Pigment 96:349–363. https://doi.org/10.1016/j.dyepig.2012.08.020
Samaher C, Mourad C, Alimi K (2021) Theoretical investigations about the effect of electron-withdrawing groups on proprieties of A-π-D- π-A type small molecules donor for organic solar cells. J Mol Model 27:54. https://doi.org/10.1007/s00894-020-04654-4
Guillén-López A, Delesma C, Amador-Bedolla C, Robles M, Muñiz J (2018) Electronic structure and nonlinear optical properties of organic photovoltaic systems with potential applications on solar cell devices: a DFT approach. Theor Chem Acc 137:1–15
Bourass M, Benzakour M, Mcharfi M, Hamidi M, Bouzzine SM, Bouachrine M (2016) DFT and TD-DFT calculation of new thienopyrazine-based small molecules for organic solar cells. Chem Central J 10:67. https://doi.org/10.1186/s13065-016-0216-6
Ramirez-Balderrama K, Orrantia-Borunda E, Flores-Holguin N (2017) Calculation of global and local reactivity descriptors of carbodiimides, a DFT study. J Theor Comput Chem 16:1750019–1750026. https://doi.org/10.1142/S0219633617500195
Alamy AE, Bourass M, Amine A, Hamidi M, Bouachrine M (2017) New organic dyes based on phenylenevinylene for solar cells: DFT and TD-DFT investigation. Karbala Int J Mod Sci 3:75–82. https://doi.org/10.1016/j.kijoms.2017.03.002
El Jouad Z, Cattin L, Addou M, Bernède JC (2019) Open circuit voltage of organic photovoltaic cells using C60 as acceptor: variation with the donor. EPJ Appl Phys 86:20201–20204. https://doi.org/10.1051/epjap/2019190047
Zhang L, Shen W, He R, Liu X, Tang X, Yang Y, Li M (2016) Fine structural tuning of diketopyrrolopyrrole-cored donor materials for small molecule-fullerene organic solar cells: A theoretical study. Org Electron 32:134–144
Lu T, Chen F (2012) Atomic dipole corrected Hirshfeld population method. J Theor Comput Chem 11:163–183. https://doi.org/10.1142/S0219633612500113
Zara Z, Iqbal J, BiBi S, Sadaf S, Eliasson B (2017) Designing benzodithiophene-based donor materials with favourable photovoltaic parameters for bulk heterojunction organic solar cells. ChemistrySelect 2:5628–5639. https://doi.org/10.1002/slct.201701008
Gierschner J, Cornil J, Egelhaaf HJ (2007) Optical bandgaps of π-conjugated organic materials at the polymer limit: experiment and theory. Adv Mater 19:173–191. https://doi.org/10.1002/adma.200600277
Acknowledgements
This work benefitted from the research modernization allowance offered by the Ministry of Higher Education of Cameroon to faculty members, for which the authors are grateful.
Author information
Authors and Affiliations
Contributions
HT: conception, design of the study, and drafting of the manuscript; BFK: revision, editing of the manuscript, providing valuable discussions, and reading the final version of the manuscript; ADTF: conception, design of the study, and reading the final version of the manuscript; SNT: editing of the manuscript and reading the final version of the manuscript; CBNT: provided valuable discussions and read the final version of the manuscript; JNG: supervision and coordination.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
Find in the supporting information the cartesian coordinates of the gas phase optimised geometry of the investigated compounds, the frequency of vibration of some specific bonds and bonding parameters as well as the aromaticity of the different rings. Also presented are the ADCH fragment charges and the absorption spectra data of the investigated molecules in gas and solution phase. (DOCX 165 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tendongmo, H., Kogge, B.F., Tamafo Fouegue, A.D. et al. Theoretical screening of N-[5′-methyl-3′-isoxasolyl]-N-[(E)-1-(-2-thiophene)] methylidene]amine and its isoxazole based derivatives as donor materials for bulk heterojunction organic solar cells: DFT and TD-DFT investigation. J Mol Model 30, 176 (2024). https://doi.org/10.1007/s00894-024-05978-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05978-1