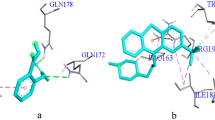
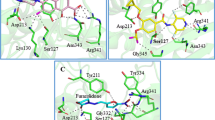
Abstract
Context
Staphylococcus aureus is a highly pathogenic organism that is the most common cause of postoperative complications as well as severe infections like bacteremia and infective endocarditis. By mediating the formation of biofilms and the expression of virulent genes, the quorum sensing (QS) mechanism is a major contributor to the development of these diseases. By hindering its QS network, an innovative approach to avoiding this bacterial infection is taken. Targeting the AgrA of the Agr system serves as beneficial in holding the top position in the QS system cascade.
Methods
Using known AgrA inhibitors, the machine learning algorithms (artificial neural network, naïve Bayes, random forest, and support vector machine) and pharmacophore model were developed. The potential lead compounds were screened against the Zinc and COCONUT databases using the best pharmacophore hypothesis. The hits were then subjected second screening process using the best machine learning model. The predicted active compounds were then reranked based on the docking score. The stability of AgrA-lead compounds was studied using molecular dynamics approaches, and an ADME profile was also carried out. Five lead compounds, namely, CNP02386963,4,5-trihydroxy-2-[({7,13,14-trihydroxy-3,10-dioxo-2,9-dioxatetracyclo[6.6.2.04,16.011,15]hexadeca-1(14),4,6,8(16),11(15),12-hexaen-6-yl}oxy)methyl]benzoic acid, CNP0129274 4-(dimethylamino)-1,5,6,10,12,12a-hexahydroxy-6-methyl-3,11-dioxo-3,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide, CNP0242717 3-Hydroxyasebotin, CNP0361624 3,4,5-trihydroxy-6-[(2,4,5,6,7-pentahydroxy-1-oxooctan-3-yl)oxy]oxane-2-carboxylic acid, and CNP0285058 2-{[4,5-dihydroxy-6-(hydroxymethyl)-3-[(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy]oxan-2-yl]oxy}-2-(4-hydroxyphenyl)acetonitrile were obtained using the two-step virtual screening process. The molecular dynamics study revealed that the CNP0238696 was found to be stable in the binding pocket of AgrA. ADME profiles show that this compound has two Lipinski violations and low bioavailability. Further studies should be performed to assess the anti-biofilm activity of the lead compound in vitro.










Similar content being viewed by others
References
Christaki E, Marcou M, Tofarides A (2020) Antimicrobial resistance in bacteria: mechanisms, evolution, and persistence. J Mol Evol 88:26–40
Lee JH (2019) Perspectives towards antibiotic resistance: from molecules to population. J Microbiol (Seoul, Korea) 57:181–184
Liu W, Yang C, Gao R, Zhang C, Ou-Yang W, Feng Z, Zhang C, Pan X, Huang P, Kong D, Wang W (2021) Polymer composite sponges with inherent antibacterial, hemostatic, inflammation-modulating and proregenerative performances for methicillin-resistant Staphylococcus aureus -infected wound healing. Adv Healthcare Mater 10:2101247
GBD (2019) Antimicrobial Resistance Collaborators (2023). Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England) 400:2221–2248
Idrees M, Sawant S, Karodia N, Rahman A (2021) Staphylococcus aureus biofilm: morphology, genetics, pathogenesis and treatment strategies. Int J Environ Res Public Health 18:7602
Ganesh PS, Veena K, Senthil R, Iswamy K, Ponmalar EM, Mariappan M, Girija ASS, Vadivelu V, Nagarajan S, Challabathula D, Shankar EM (2022) Biofilm-associated Agr and Sar quorum sensing systems of Staphylococcus aureus are inhibited by 3-hydroxybenzoic acid derived from Illicium verum. ACS Omega 7:14653–14665
Lade, Chung SH, Lee Y, Kumbhar BV, Joo H-S, Kim Y-G, Yang Y-H, Kim J-S (2022) Thymol reduces agr-mediated virulence factor phenol-soluble modulin production in staphylococcus aureus. BioMed Research International 8221622
Rajasree K, Fasim A, Gopal B (2016) Conformational features of the Staphylococcus aureus AgrA-promoter interactions rationalize quorum-sensing triggered gene expression. Biochem Biophys Reports 6:124–134
Iobbi V, Brun P, Bernabé G, DouguéKentsop RA, Donadio G, Ruffoni B, Fossa P, Bisio A, De Tommasi N (2021) Labdane Diterpenoids from Salvia tingitana Etl. Synergize with Clindamycin against methicillin-resistant Staphylococcus aureus. Molecules (Basel, Switzerland) 26:6681
Leonard PG, Bezar IF, Sidote DJ, Stock AM (2012) Identification of a hydrophobic cleft in the LytTR domain of AgrA as a locus for small molecule interactions that inhibit DNA binding. Biochemistry 51:10035–10043
Wang B, Muir TW (2016) Regulation of virulence in Staphylococcus aureus: molecular mechanisms and remaining puzzles. Cell Chem Biol 23:214–224
Bernabè G, Dal Pra M, Ronca V, Pauletto A, Marzaro G, Saluzzo F, Stefani A, Artusi I, De Filippis V, Ferlin MG, Brun P, Castagliuolo I (2021) A novel Aza-derivative inhibits agr quorum sensing signaling and synergizes methicillin-resistant Staphylococcus aureus to clindamycin. Front Microbiol 12:610859
Greenberg M, Kuo D, Jankowsky E, Long L, Hager C, Bandi K, Ma D, Manoharan D, Shoham Y, Harte W, Ghannoum MA, Shoham M (2018) Small-molecule AgrA inhibitors F12 and F19 act as antivirulence agents against Gram-positive pathogens. Sci Rep 8:14578
Palaniappan B, Solomon AP, C DR, (2021) Targeting AgrA quorum sensing regulator by bumetanide attenuates virulence in Staphylococcus aureus - a drug repurposing approach. Life Sci 273:119306
Mahdally NH, George RF, Kashef MT, Al-Ghobashy M, Murad FE, Attia AS (2021) Staquorsin: a novel Staphylococcus aureus Agr-mediated quorum sensing inhibitor impairing virulence in vivo without notable resistance development. Front Microbiol 12:700494
Sully EK, Malachowa N, Elmore BO, Alexander SM, Femling JK, Gray BM, DeLeo FR, Otto M, Cheung AL, Edwards BS, Sklar LA, Horswill AR, Hall PR, Gresham HD (2014) Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog 10:e1004174
Kumari M, Tiwari N, Chandra S, Subbarao N (2018) Comparative analysis of machine learning based QSAR models and molecular docking studies to screen potential anti-tubercular inhibitors against InhA of mycobacterium tuberculosis. Int J Comput Biol Drug Des 11:209
Liang JW, Wang MY, Wang S, Li SL, Li WQ, Meng FH (2020) Identification of novel CDK2 inhibitors by a multistage virtual screening method based on SVM, pharmacophore and docking model. J Enzyme Inhib Med Chem 35:235–244
Hall M, Frank E, Holmes G, Pfahringer B, Reutemann P, Witten IH (2009) The WEKA Data Mining Software: an update. ACM SIGKDD Explorations Newsl 11:10
Zupan J, Gasteiger J (1993) Neural networks for chemists; an introduction. VCH Publishers, Weinheim (Germany)
Friedman N, Geiger D, Goldszmidt M (1997) Bayesian network classifiers. Mach Learn 29:131–163. https://doi.org/10.1023/A:1007465528199
Breiman L (2001) Random forest. Mach Learn 45:5–32
Vapnik VN (1998) Statistical Learning Theory. Wiley
Schrödinger Release 2022-3 (2022) Schrödinger Release 2022-3: Phase, Schrödinger, LLC, New York
Misra S, Saini M, Ojha H, Sharma D, Sharma K (2017) Pharmacophore modelling, atom-based 3D-QSAR generation and virtual screening of molecules projected for mPGES-1 inhibitory activity. SAR QSAR Environ Res 28:17–39
Dixon SL, Smondyrev AM, Knoll EH, Rao SN, Shaw DE, Friesner RA (2006) PHASE: a new engine for pharmacophore perception, 3D QSAR model development, and 3D database screening: 1. Methodology and preliminary results. J Comput Aided Mol Des 20:647–671
Schrödinger Release 2022-3 (2022) Schrödinger Release 2022-3: Glide, Schrödinger, LLC, New York, NY
Schrödinger Release 2022-3 (2022) Schrödinger Release 2022-3: LigPrep, Schrödinger, LLC, New York, NY
Schrödinger Release 2022-3 (2022) Schrödinger Release 2022-3: Protein Preparation Wizard; Epik, Schrödinger, LLC, New York, NY
Schrödinger Release 2022-3 (2022) Schrödinger Release 2022-3: SiteMap, Schrödinger, LLC, New York, NY
Schrödinger Release 2022-3 (2022) Schrödinger Release 2022-3: Prime, Schrödinger, LLC, New York, NY
Schrödinger Release 2020-4 (2020) Schrödinger Release 2020-4: Desmond Molecular Dynamics System. D. E. Shaw Research. Maestro-Desmond Interoperability Tools, Schrödinger, New York NY
Schrödinger Release 2022-3 (2022) Schrödinger Release 2022-3: QikProp, Schrödinger, LLC, New York, NY
Opo FADM, Rahman MM, Ahammad F, Ahmed I, Bhuiyan MA, Asiri AM (2021) Structure based pharmacophore modeling, virtual screening, molecular docking and ADMET approaches for identification of natural anti-cancer agents targeting XIAP protein. Sci Rep 11:4049
Jamal S, Grover A, Grover S (2019) Machine learning from molecular dynamics trajectories to predict caspase-8 inhibitors against Alzheimer’s disease. Front Pharmacol 10:780
Mishra S, Mallick PK, Tripathy HK, Bhoi AK, González-Briones A (2020) Performance evaluation of a proposed machine learning model for chronic disease datasets using an integrated attribute evaluator and an improved decision tree classifier. Appl Sci 10(22):8137
Sarker IH, Kayes ASM, Watters P (2019) Effectiveness analysis of machine learning classification models for predicting personalized context-aware smartphone usage. J Big Data 6:57
Chicco D, Jurman G (2020) The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genomics 21:6
Hdoufane L, Stoycheva J, Tadjer A, Villemin D, Najdoska-Bogdanov M, Bogdanov J, Cherqaoui D (2019) QSAR and molecular docking studies of indole-based analogs as HIV-1 attachment inhibitors. J Mol Struct 1193:429–443
Giordano D, Biancaniello C, Argenio MA, Facchiano A (2022) Drug design by pharmacophore and virtual screening approach. Pharmaceuticals (Basel, Switzerland) 15:646
Srivastava SK, Rajasree K, Fasim A, Arakere G, Gopal B (2014) Influence of the AgrC-AgrA complex on the response time of Staphylococcus aureus quorum sensing. J Bacteriol 196:2876–2888
Daly SM, Elmore BO, Kavanaugh JS, Triplett KD, Figueroa M, Raja HA, El-Elimat T, Crosby HA, Femling JK, Cech NB, Horswill AR, Oberlies NH, Hall PR (2015) ω-Hydroxyemodin limits staphylococcus aureus quorum sensing-mediated pathogenesis and inflammation. Antimicrob Agents Chemother 59:2223–2235
Paul P, Chakraborty P, Sarker RK, Chatterjee A, Maiti D, Das A, Mandal S, Bhattacharjee S, Dastidar DG, Tribedi P (2021) Tryptophan interferes with the quorum sensing and cell surface hydrophobicity of Staphylococcus aureus: a promising approach to inhibit the biofilm development. 3 Biotech 11:376
Nazar A, Abbas G, Azam SS (2020) Deciphering the inhibition mechanism of under trialHsp90 inhibitors and their analogues: a comparative molecular dynamics simulation. J Chem Inf Model 60:3812–3830
Pal S, Kumar V, Kundu B, Bhattacharya S, Preethy N, Reddy MP, Taulkdar A (2019) Ligand-based pharmacophore modelling, virtual screening and molecular docking studies for discovery of potential topoisomerase I inhibitors. Comput Struct Biotechnol J 17:291–310
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23:3–25
Shahbazi S, Kuanar A, Gade DR, Kar D, Shrivastava A, Kunala P, Mahto MK (2016) Semiemperical investigation of the postmenopausal breast cancer treatment potential of xanthone derivatives. Nat Prod Chem Res 4:206
Arora R, Issar U, Kakkar R (2019) Identification of novel urease inhibitors: pharmacophore modeling, virtual screening and molecular docking studies. J Biomol Struct Dyn 37:4312–4326
Kumar A, Sahoo SK, Padhee K, Kochar P, Satapathy A, Pathak N (2011) Review on solubility enhancement techniques for hydrophobic drugs. Pharm Glob: Int J Compr Pharm 2:001–007
Acknowledgements
The authors are grateful to the Centre for Machine Learning and Intelligence (CMLI) at Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore, Tamil Nadu, India, for their continuous rendering of and support during the work.
Funding
This work was supported by the DST-CURIE-AI-PhaseII (Project No. 16), Centre for Machine Learning and Intelligence (CMLI) at Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore, Tamil Nadu, India.
Author information
Authors and Affiliations
Contributions
Conception and design: Rajeswari Murugesan. Development of methodology and acquisition of data: Monica Ramasamy and Rajeswari Murugesan, Analysis; interpretation of data: Monica Ramasamy, Aishwarya Vetrivel, and Rajeswari Murugesan; writing, review, and/or revision of the manuscript: Monica Ramasamy, Aishwarya Vetrivel, Sharulatha Venugopal, and Rajeswari Murugesan.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramasamy, M., Vetrivel, A., Venugopal, S. et al. Identification of inhibitors for Agr quorum sensing system of Staphylococcus aureus by machine learning, pharmacophore modeling, and molecular dynamics approaches. J Mol Model 29, 258 (2023). https://doi.org/10.1007/s00894-023-05647-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05647-9