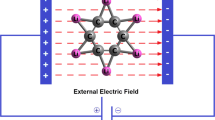
Abstract
Herein, we examined the nonlinear optical properties of thia[7&8]circulenes (1–18). Circulenes are the building blocks of various nanomaterials such as graphene, nanotubes, and fullerenes. Many studies on circulenes have focused on the aromaticity of circulenes, but less attention has been paid on optoelectronics properties. Carbon atoms of the [7&8]circulenes are replaced with multiple sulfur atoms to designed thia[7&8]circulenes (1–18). These circulenes (1–18) are thermodynamically, kinetically, and chemically stable. Nonlinear optical (NLO) response is evaluated through static and frequency-dependent first and second hyperpolarizability values. The static first hyperpolarizability (βo) of these compounds ranges between 0.00 and 496 au. The frequency-dependent coefficients for all thia[7&8]circulenes show remarkable enhancement at 532 and 1064 nm, respectively. The nonlinear refractive index is increased up to 1.13 × 10−14 au for circulene 9 at 532 nm. These findings successfully demonstrated that nonlinear optical response of thia[7&8]circulenes can be increased by decorating multiple sulfur atoms. The unsymmetrical distribution of sulfur atoms is more effective in enhancing nonlinear optical response.




Similar content being viewed by others
Code availability
The software used is commercially available.
References
Kanis DR, Ratner MA, Marks TJ (1994) Design and construction of molecular assemblies with large second-order optical nonlinearities. Quantum chemical aspects. Chem Rev 94:195–242. https://doi.org/10.1021/cr00025a007
Oudar JL, Chemla DS (1977) Hyperpolarizabilities of the nitroanilines and their relations to the excited state dipole moment. J Chem Phys 66:2664–2668. https://doi.org/10.1063/1.434213
Coe BJ (2006) Switchable nonlinear optical metallochromophores with pyridinium electron acceptor groups. Acc Chem Res 39:383–393. https://doi.org/10.1021/ar050225k
Muhammad S, Xu H-L, Zhong R-L, Su Z-M, Al-Sehemi AG, Irfan A (2013) Quantum chemical design of nonlinear optical materials by sp2-hybridized carbon nanomaterials: issues and opportunities. J Mater Chem C 1:5439. https://doi.org/10.1039/c3tc31183j
Blanchard-Desce M, Alain V, Bedworth PV, Marder SR, Fort A, Runser C, Barzoukas M, Lebus S, Wortmann R (1997) Large quadratic hyperpolarizabilities with donor–acceptor polyenes exhibiting optimum bond length alternation: correlation between structure and hyperpolarizability. Chem - A Eur J 3:1091–1104. https://doi.org/10.1002/chem.19970030717
Zhang C, Song Y, Wang X (2007) Correlations between molecular structures and third-order non-linear optical functions of heterothiometallic clusters: a comparative study. Coord Chem Rev 251:111–141. https://doi.org/10.1016/j.ccr.2006.06.007
Sang HL, Jo RP, Jeong MY, Hwan MK, Li S, Song J, Ham S, Jeon SJ, Bong RC (2006) First hyperpolarizabilities of 1,3,5-tricyanobenzene derivatives: origin of larger β values for the octupoles than for the dipoles. ChemPhysChem 7:206–212. https://doi.org/10.1002/cphc.200500274
Liu CG, Guan W, Song P, Yan LK, Su ZM (2009) Redox-switchable second-order nonlinear optical responses of push-pull monotetrathiafulvalene-metalloporphyrins. Inorg Chem 48:6548–6554. https://doi.org/10.1021/ic9004906
Wu C-L, Lin Y-H, Su S-P, Huang B-J, Tsai C-T, Wang H-Y, Chi Y-C, Wu C-I, Lin G-R (2015) Enhancing optical nonlinearity in a nonstoichiometric SiN waveguide for cross-wavelength all-optical data processing. ACS Photonics 2:1141–1154. https://doi.org/10.1021/acsphotonics.5b00192
Hsieh C-H, Chou L-J, Lin G-R, Bando Y, Golberg D (2008) Nanophotonic switch: gold-in-Ga 2 O 3 peapod nanowires. Nano Lett 8:3081–3085. https://doi.org/10.1021/nl0731567
Marder SR, Perry JW (1993) Molecular materials for second-order nonlinear optical applications. Adv Mater 5:804–815. https://doi.org/10.1002/adma.19930051104
Cagardová D, Matúška J, Poliak P, Lukeš V (2019) Design of novel generations of planar sunflower molecules: theoretical comparative study of electronic structure and charge transport characteristics. J Phys Chem C 123:22752–22766. https://doi.org/10.1021/acs.jpcc.9b05598
Dopper JH, Wynberg H (1972) Heterocirculenes a new class of polycyclic aromatic hydrocarbons. Tetrahedron Lett 13:763–766. https://doi.org/10.1016/S0040-4039(01)84432-4
Fujimoto T, Matsushita MM, Awaga K (2010) Dual-gate field-effect transistors of octathio[8]circulene thin-films with ionic liquid and SiO2 gate dielectrics. Appl Phys Lett 97:123303. https://doi.org/10.1063/1.3491807
Baryshnikov GV, Minaev BF, Minaeva VA (2015) Electronic structure, aromaticity and spectra of hetero[8]circulenes. Russ Chem Rev 84:455–484. https://doi.org/10.1070/RCR4445
Nielsen CB, Brock-Nannestad T, Reenberg TK, Hammershøj P, Christensen JB, Stouwdam JW, Pittelkow M (2010) Organic light-emitting diodes from symmetrical and unsymmetrical π-extended tetraoxa[8]circulenes. Chem - A Eur J 16:13030–13034. https://doi.org/10.1002/chem.201002261
Burn PL, Lo S-C, Samuel IDW (2007) The development of light-emitting dendrimers for displays. Adv Mater 19:1675–1688. https://doi.org/10.1002/adma.200601592
Fujimoto T, Matsushita MM, Awaga K (2012) Ionic-liquid component dependence of carrier injection and mobility for electric-double-layer organic thin-film transistors. J Phys Chem C 116:5240–5245. https://doi.org/10.1021/jp2122642
Minaev BF, Baryshnikov GV, Minaeva VA (2011) Density functional theory study of electronic structure and spectra of tetraoxa[8]circulenes, Comput. Theor Chem 972:68–74. https://doi.org/10.1016/j.comptc.2011.06.020
Yu J, Sun Q, Kawazoe Y, Jena P (2014) Stability and properties of 2D porous nanosheets based on tetraoxa[8]circulene analogues. Nanoscale 6:14962–14970. https://doi.org/10.1039/C4NR05037A
Kuklin AV, Baryshnikov GV, Minaev BF, Ignatova N, Ågren H (2018) Strong topological states and high charge carrier mobility in tetraoxa[8]circulene nanosheets. J Phys Chem C 122:22216–22222. https://doi.org/10.1021/acs.jpcc.8b08596
Chen F, Tanaka T, Mori T, Osuka A (2018) Synthesis, structures, and optical properties of azahelicene derivatives and unexpected formation of azahepta[8]circulenes. Chem A Eur J 24:7489–7497
Baryshnikov GV, Valiev RR, Karaush NN, Minaeva VA, Sinelnikov AN, Pedersen SK, Pittelkow M, Minaev BF, Ågren H (2016) Benzoannelated aza-, oxa- and azaoxa[8]circulenes as promising blue organic emitters. Phys Chem Chem Phys 18:28040–28051. https://doi.org/10.1039/C6CP03060B
Kato S, Akahori S, Serizawa Y, Lin X, Yamauchi M, Yagai S, Sakurai T, Matsuda W, Seki S, Shinokubo H, Miyake Y (2020) Systematic synthesis of tetrathia[8]circulenes: the influence of peripheral substituents on the structures and properties in solution and solid states. J Org Chem 85:62–69. https://doi.org/10.1021/acs.joc.9b01655
Mousavi M, Fini EH (2021) Phenolic compounds to amplify the effect of sulfur on Bitumen’s thermomechanical properties. Fuel 287:119532. https://doi.org/10.1016/j.fuel.2020.119532
Oftadeh M, Naseh S, Hamadanian M (2011) Electronic properties and dipole polarizability of thiophene and thiophenol derivatives via density functional theory, Comput. Theor Chem 966:20–25. https://doi.org/10.1016/j.comptc.2011.02.003
Al‐Janabi ASM, Al‐Samra UAY, Othman EA, Yousef TA (2020) Optical properties, structural, and DFT studies of Pd(II) complexes with 1‐phenyl‐1 H‐tetrazol‐5‐thiol, X‐ray crystal structure of [Pd(κ1‐S‐ptt) 2 (κ2‐dppe)] complex. Appl Organomet Chem 34:e5996. https://doi.org/10.1002/aoc.5996
Narita A, Wang X-Y, Feng X, Müllen K (2015) New advances in nanographene chemistry. Chem Soc Rev 44:6616–6643. https://doi.org/10.1039/C5CS00183H
Stępień M, Gońka E, Żyła M, Sprutta N (2017) Heterocyclic nanographenes and other polycyclic heteroaromatic compounds: synthetic routes, properties, and applications. Chem Rev 117:3479–3716. https://doi.org/10.1021/acs.chemrev.6b00076
Yoshida K, Osuka A (2015) Peripherally hexasulfanylated subporphyrins. Chem - An Asian J 10:1526–1534. https://doi.org/10.1002/asia.201500225
Chernichenko KY, Balenkova ES, Nenajdenko VG (2008) From thiophene to sulflower. Mendeleev Commun 18:171–179. https://doi.org/10.1016/j.mencom.2008.07.001
Gingras M, Pinchart A, Dallaire C, Mallah T, Levillain E (2004) Star-shaped nanomolecules based onp-Phenylene Sulfide Asterisks with a persulfurated coronene Core. Chem - A Eur J 10:2895–2904. https://doi.org/10.1002/chem.200305605
Chernichenko KY, Sumerin VV, Shpanchenko RV, Balenkova ES, Nenajdenko VG (2006) “Sulflower”: a new form of carbon sulfide, Angew. Chemie 118:7527–7530. https://doi.org/10.1002/ange.200602190
Datta A, Pati SK (2007) Computational design of high hydrogen adsorption efficiency in molecular “sulflower.” J Phys Chem C 111:4487–4490. https://doi.org/10.1021/jp070609n
Dong R, Pfeffermann M, Skidin D, Wang F, Fu Y, Narita A, Tommasini M, Moresco F, Cuniberti G, Berger R, Müllen K, Feng X (2017) Persulfurated coronene: a new generation of “sulflower.” J Am Chem Soc 139:2168–2171. https://doi.org/10.1021/jacs.6b12630
Mahmood T, Kosar N, Ayub K (2017) DFT study of acceleration of electrocyclization in photochromes under radical cationic conditions: comparison with recent experimental data. Tetrahedron 73:3521–3528. https://doi.org/10.1016/j.tet.2017.05.031
Ullah F, Kosar N, Ayub K, Mahmood T (2019) Superalkalis as a source of diffuse excess electrons in newly designed inorganic electrides with remarkable nonlinear response and deep ultraviolet transparency: a DFT study. Appl Surf Sci 483:1118–1128. https://doi.org/10.1016/j.apsusc.2019.04.042
Kosar N, Mahmood T, Ayub K, Tabassum S, Arshad M, Gilani MA (2019) Do** superalkali on Zn12O12 nanocage constitutes a superior approach to fabricate stable and high-performance nonlinear optical materials. Opt Laser Technol 120:105753. https://doi.org/10.1016/j.optlastec.2019.105753
Kosar N, Tahir H, Ayub K, Gilani MA, Arshad M, Mahmood T (2021) Impact of even number of alkaline earth metal do** on the NLO response of C20 nanocluster; a DFT outcome. Comput Theor Chem 1204:113386. https://doi.org/10.1016/j.comptc.2021.113386
Kosar N, Ayub K, Gilani MA, Shah F, Mahmood T (2020) Benchmark approach to search of cost‐effective and accurate density functional for homolytic cleavage of C─Mg bond of Grignard reagent. Int J Quantum Chem 120:e2606. https://doi.org/10.1002/qua.26106
Rudnitskaya A, Török B, Dransfield T (2011) A B3LYP/6-31+G(d) study of the reaction pathways and conformational preference in a model Chichibabin reaction, Comput. Theor Chem 963:191–199. https://doi.org/10.1016/j.comptc.2010.10.023
Muzomwe M, Maes G, Kasende OE (2012) Theoretical DFT(B3LYP)/6-31+G(d) study on the prediction of the preferred interaction site of 3-methyl-4-pyrimidone with different proton donors. Nat Sci 04:286–297. https://doi.org/10.4236/ns.2012.45041
Kosar N, Ayub K, Gilani MA, Mahmood T (2019) Benchmark DFT studies on C-CN homolytic cleavage and screening the substitution effect on bond dissociation energy. J Mol Model 25:47. https://doi.org/10.1007/s00894-019-3930-x
Alparone A (2011) Comparative study of CCSD(T) and DFT methods: electronic (hyper)polarizabilities of glycine. Chem Phys Lett 514:21–25. https://doi.org/10.1016/j.cplett.2011.08.010
Jacquemin D, Adamo C (2011) Bond length alternation of conjugated oligomers: wave function and DFT benchmarks. J Chem Theory Comput 7:369–376. https://doi.org/10.1021/ct1006532
Alparone A (2013) Linear and nonlinear optical properties of nucleic acid bases. Chem Phys 410:90–98. https://doi.org/10.1016/j.chemphys.2012.11.005
Li X, Lu J (2019) Giant enhancement of electronic polarizability and the first hyperpolarizability of fluoride-decorated graphene versus graphyne and graphdiyne: insights from ab initio calculations. Phys Chem Chem Phys 21:13165–13175. https://doi.org/10.1039/C9CP01118H
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G (2010) Gaussian 09, Rev. B. 01. Gaussian Inc., Wallingford
Dennington R, Todd K, John M (2009) Gauss view, version 5. Semichem Inc., Shawnee Mission, KS, USA
Yang Y, Helili S, Kerim A (2022) A study of the aromaticity of thia[7]circulene isomers. Mol Phys 120:10. https://doi.org/10.1080/00268976.2022.2060146
Liljefors T, Wennerström O (1977) Molecular mechanics calculations on the structures and conformational properties of [8]circulene and some related phane compounds. Tetrahedron 33:2999–3003. https://doi.org/10.1016/0040-4020(77)88037-X
**ao WD, Zhang YY, Tao L, Aït-Mansour K, Chernichenko KY, Nenajdenko VG, Ruffieux P, Du SX, Gao H-J, Fasel R (2015) Impact of heterocirculene molecular symmetry upon two-dimensional crystallization. Sci Rep 4:5415. https://doi.org/10.1038/srep05415
Agrawal BK, Agrawal S, Srivastava P, Singh S (2004) Ab-initio study of small silver nanoclusters. J Nanoparticle Res 6:363–368. https://doi.org/10.1023/B:NANO.0000046744.53994.4d
Sumathi A, Rajaraman D, Bharanidharan S, Kabilan S, Krishnasamy K (2016) Spectroscopic investigations of 2-(4-chlorophenyl)-1-((furan-2-yl) methyl)-4,5-dimethyl-1H-imidazole - a DFT approach. Chem Sci Trans 5(3):827–835
Zaier R, Ayachi S (2021) DFT molecular modeling studies of D-π-A-π-D type cyclopentadithiophene-diketopyrrolopyrrole based small molecules donor materials for organic photovoltaic cells. Optik (Stuttg) 239:166787. https://doi.org/10.1016/j.ijleo.2021.166787
Arab A, Habibzadeh M (2015) Comparative hydrogen adsorption on the pure Al and mixed Al–Si nano clusters: a first principle DFT study, Comput. Theor Chem 1068:52–56. https://doi.org/10.1016/j.comptc.2015.06.021
Shakerzadeh E (2018) Tailoring C24S12 and C16S8 sulflowers with lithium atom for the remarkable first hyperpolarizability. Chem Phys Lett 709:33–40. https://doi.org/10.1016/j.cplett.2018.08.039
Kang S, Kim T, Lee JY (2021) New design strategy for chemically-stable blue phosphorescent materials: improving the energy gap between the T 1 and 3 MC states. Phys Chem Chem Phys 23:3543–3551. https://doi.org/10.1039/C9CP05781A
Cagardová D, Lukeš V, Matúška J, Poliak P (2019) On local aromaticity of selected model aza-[n]circulenes (n = 6, 7, 8 and 9): density functional theoretical study. Acta Chim Slovaca 12:70–81. https://doi.org/10.2478/acs-2019-0011
Irfan A, Al-Sehemi AG (2014) DFT study of the electronic and charge transfer properties of perfluoroarene–thiophene oligomers. J Saudi Chem Soc 18:574–580. https://doi.org/10.1016/j.jscs.2011.11.006
Yildiko Ü, Ata AC, Cakmak İ (2020) Synthesis, spectral characterization and DFT calculations of novel macro MADIX agent: mechanism of addition-fragmentation reaction of xanthate compound. SN Appl Sci 2:1691. https://doi.org/10.1007/s42452-020-03495-3
Mumit MA, Pal TK, Alam MA, Islam MAAAA, Paul S, Sheikh MC (2020) DFT studies on vibrational and electronic spectra, HOMO–LUMO, MEP, HOMA, NBO and molecular docking analysis of benzyl-3-N-(2,4,5-trimethoxyphenylmethylene)hydrazinecarbodithioate. J Mol Struct 1220:128715. https://doi.org/10.1016/j.molstruc.2020.128715
Murugavel S, Vetri Velan V, Kannan D, Bakthadoss M (2017) Synthesis of a novel methyl(2E)-2-{[N-(2-formylphenyl)(4-methylbenzene) sulfonamido]methyl}-3-(2-methoxyphenyl)prop-2-enoate: molecular structure, spectral, antimicrobial, molecular docking and DFT computational approaches. J Mol Struct 1127:457–475. https://doi.org/10.1016/j.molstruc.2016.08.001
Tarazkar M, Romanov DA, Levis RJ (2014) Higher-order nonlinearity of refractive index: the case of argon. J Chem Phys 140:214316. https://doi.org/10.1063/1.4880716
Bree C, Demircan A, Steinmeyer G (2010) Method for computing the nonlinear refractive index via Keldysh theory. IEEE J Quantum Electron 46:433–437. https://doi.org/10.1109/JQE.2009.2031599
Funding
Financial support for this project was provided by the Higher Education Commission of Pakistan, COMSATS University, Abbottabad Campus, and University of Bahrain.
Author information
Authors and Affiliations
Contributions
Naveen Kosar, acquisition and analysis of data and manuscript drafting; Muhammad Arshad, acquisition and analysis of data; Mazhar Amjad Gilani and Khurshid Ayub, study conception and design and critical revision; and Tariq Mahmood, supervision, study conception and design, and critical revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
NA
Consent to participate
NA
Consent for publication
All authors agree for the publication of the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kosar, N., Ayub, K., Gilani, M.A. et al. Shedding light on static and dynamic hyperpolarizabilities of thia[7&8]circulenes, toward their NLO applications. J Mol Model 28, 395 (2022). https://doi.org/10.1007/s00894-022-05386-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05386-3