Abstract
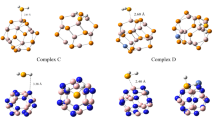
In this research, we have reported the electrical sensitivity of pristine C60 and silicon doped on C60 (SiC59) nanocages as sensors that can be used for detecting the presence of alkali (Li+, Na+, K+) and alkaline earth (Be2+, Mg2+, Ca2+) cations. The computations are carried out at the B3LYP level of theory with a 6-31G(d) basis set. The atoms in molecules (AIM) and natural bond orbital (NBO) analyses are performed to evaluate the intermolecular interactions between cations and nanocages. The physical properties of the selected complexes are also analyzed by the frontier molecular orbital, energy gap, electronegativity, chemical hardness, softness, and other quantities such as work function, number of transferred electron, and dipole moment. The results show that the adsorption process is exothermic and with increasing the charge of cations, the adsorption energies enhance. Our findings also reveal a decrease in the energy gap along with an increase in the electrical conductivity of the respective complexes. Finally, the density of state calculations is presented to confirm the obtained results.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
Code availability
Not applicable.
References
Kroto W, Heath JR, O’Brien SC, Curl RF, Smalley RE (1985) C60: buckminsterfullerene. Nature 318:162–163
Zahedi E (2013) Adsorption of nitrogen dioxide on C30B15N15 heterofullerene: AIM and NBO study via DFT. C R Chimie 16:189–194
Lalwani G, Sitharaman B (2013) Multifunctional fullerene and metallofullerene based nanobiomaterials. Nano LIFE 3:1342003
Yang M, Li C, Zhang Y, Jia D, Zhang X, Hou Y, Li R, Wang J (2017) Maximum undeformed equivalent chip thickness for ductile-brittle transition of zirconia ceramics under different lubrication conditions. Int J Mach Tools Manuf 122:55–65
Yang M, Li C, Zhang Y, Jia D, Li R, Hou Y, Cao H, Wang J (2019) Predictive model for minimum chip thickness and size effect in single diamond grain grinding of zirconia ceramics under different lubricating conditions. Ceram 45(12):14908–14920
Zhang Y, Li HN, Li C, Huang C, Ali HM, XU X, Mao C, Ding W, Cui X, Yang M, Yu T, Jamil M, Gupta MK, Jia D, Said Z (2022) Nano-enhanced biolubricant in sustainable manufacturing: from processability to mechanisms. Friction 10:803–841
Li B, Li C, Zhang Y, Wang Y, Jia D, Yang M (2016) Grinding temperature and energy ratio coefficient in MQL grinding of high-temperature nickel-base alloy by using different vegetable oils as base oil. Chinese J Aeronaut 29(4):1084–1095
Guo S, Li C, Zhang Y, Wang Y, Li B, Yang M, Zhang X, Liu G (2017) Experimental evaluation of the lubrication performance of mixtures of castor oil with other vegetable oils in MQL grinding of nickel-based alloy. J Clean Prod 140:1060–1076
Zhang J, Li C, Zhang Y, Yang M, Jia D, Liu G, Hou Y, Li R, Zhang N, Wu Q, Cao H (2018) Experimental assessment of an environmentally friendly grinding process using nanofluid minimum quantity lubrication with cryogenic air. J Clean Prod 193:236–248
Rutherglen C, Jain D, Burke P (2009) Nanotube electronics for radiofrequency applications. Nat Nanotechnol 4:811–819
Schwierz F (2010) Graphene transistors. Nat Nanotechnol 5:487–496
Motaung DE, Malgas GF, Arendse CJ (2011) Insights into the stability and thermal degradation of P3HT: C60 blended films for solar cell applications. J Mater Sci 46:4942–4952
Haddon R (1992) Electronic structure, conductivity and superconductivity of alkali metal doped (C60). Acc Chem Res 25:127–133
Vessally E, Farajzadeh P, Najafi E (2021) Possible sensing ability of boron nitride nanosheet and its Al– and Si–doped derivatives for methimazole drug by computational study. Iran J Chem Chem Eng 40:1001–1011
Vessally E, Musavi M, Poor Heravi MR (2021) A density functional theory study of adsorption ethionamide on the surface of the pristine, Si and Ga and Al-doped graphene. Iran J Chem Eng (IJCCE) 40 in Press
Vessally E, Hosseinian A (2021) A computational study on some small graphene-like nanostructures as the anodes in Na−ion Batteries. Iran J Chem Chem Eng 40:691–703
Gharibzadeh F, Vessally E, Edjlali L, Es'haghi M, Mohammadi R (2020) A DFT study on sumanene, corannulene and nanosheet as the anodes in Li−Ion batteries. Iran J Chem Chem Eng 39:51-62
Hashemzadeh B, Edjlali L, Delir Kheirollahi Nezhad P, Vessally E (2021) A DFT studies on a potential anode compound for Li-ion batteries: hexa-cata-hexabenzocoronene nanographen. Chem Rev Lett 4:232–238
Bosi S, Da Ros T, Castellano S, Banfi E, Prato M (2000) Antimycobacterial activity of ionic fullerene derivatives. Bioorg Med Chem Lett 10:1043–1045
Friedman SH, DeCamp DL, Sijbesma RP, Srdanov G, Wudl F, Kenyon GL (1993) Inhibition of the HIV-1 protease by fullerene derivatives: model building studies and experimental verification. J Am Chem Soc 115:6506–6509
Nakamura E, Tokuyama H, Yamago S, Shiraki T, Sugiura Y (1996) Biological activity of water-soluble fullerenes. Structural dependence of DNA cleavage, cytotoxicity, and enzyme inhibitory activities including HIV-protease inhibition. Bull Chem Soc Jpn 69:2143–2151
Luo B, Liu G, Wang L (2016) Recent advances in 2D materials for photocatalysis. Nanoscale 8:6904–6920
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field in atomically thin carbon films. Science 306:666–669
Chen M, Guan R, Yang S (2019) Hybrids of fullerenes and 2D nanomaterials. Adv Sci (Weinh) 6:1800941
Jalali Sarvestani MR, Charehjou P (2021) Fullerene (C20) as a potential adsorbent and sensor for the removal and detection of picric acid contaminant: a DFT Study. Cent Asian J Environ Sci Technol Innov 2(1):12–19
Onur Uygun Z, Ertuğrul Uygun HD (2020) Fullerene based sensor and biosensor technologies, In book: Nanosystems, IntechOpen
Ren XY, Jiang CY (2012) Density functional studies on the endohedral complex of fullerene C70 with tetrahedrane (C4H4): C4H4@C70. J Mol Model 18:3213–3217
Alipour Zaghmarzi F, Zahedi M, Mola A, Abedini S, Arshadi S, Ahmadzadeh S, Etminan N, Younesi O, Rahmanifar E, Yoosefian M (2017) Fullerene-C60 and crown ether doped on C60 sensors for high sensitive detection of alkali and alkaline earth cations. Physica E 87:51–58
Zheng J, Ren Z, Guo P, Fang L, Fan J (2011) Diffusion of Li+ ion on graphene: a DFT study. Appl Surf Sci 258:1651–1655
Sanghavi BJ, Varhue W, Rohani A, Liao KT, Bazydlo LA, Chou CF, Swami NS (2015) Ultrafast immunoassays by coupling dielectrophoretic biomarker enrichment in nanoslit channel with electrochemical detection on graphene. Lab Chip 15:4563–4570
Sanghavi BJ, Moore JA, Chávez JL, Hagen JA, Kelley-Loughnane N, Chou CF, Swami NS (2016) Aptamer-functionalized nanoparticles for surface immobilization-free electrochemical detection of cortisol in a microfluidic device. Biosens Bioelectron 78:244–252
Sanghavi BJ, Varhue W, Chávez JL, Chou CF, Swami NS (2014) Electrokinetic preconcentration and detection of neuropeptides at patterned graphene-modified electrodes in a nanochannel. Anal Chem 86:4120–4125
Zhang X, Tang Y, Zhang F, Lee C (2016) A novel aluminum-graphite dual-ion battery. Adv Energy Mater 6(11):1502588
Tong X, Zhang F, Ji B, Sheng M, Tang Y (2016) carbon-coated porous aluminum foil anode for high-rate, long-term cycling stability, and high energy density dual-ion batteries. Adv Mater (Weinh) 28(45):9979–9985
Ji B, Zhang F, Song X, Tang Y (2017) A novel potassium-ion-based dual-ion battery. Adv Mater (Weinh) 29(19):1700519
Wang M, Jiang C, Zhang S, Song X, Tang Y, Cheng HM (2018) Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat Chem 10(6):667–672
Jiang L, Wang Y, Wang X, Ning F, Wen S, Zhou Y, Chen S, Betts A, Jerrams S, Zhou F (2021) Electrohydrodynamic printing of a dielectric elastomer actuator and its application in tunable lenses. Compos-A: Appl Sci Manuf 147:106461
Li T, Yin W, Gao S, Sun Y, Xu P, Wu S, Kong H, Yang G, Wei G (2022) The combination of two-dimensional nanomaterials with metal oxide nanoparticles for gas sensors: a review. Nanomaterials 12(6):982
Yan H, Zhao M, Feng X, Zhao S, Zhou X, Li S, Zha M, Meng F, Chen X, Liu Y, Chen D, Yan N, Yang C (2022) PO43- coordinated robust single-atom platinum catalyst for selective polyol oxidation. Angew Chem Int Ed e202116059
Zhang R, Zhang W, Shi M, Li H, Ma L, Niu H (2022) Morphology controllable synthesis of heteroatoms-doped carbon materials for high-performance flexible supercapacitor. Dyes Pigm 199:109968
Huang Z, Luo P, Zheng H (2022) Design of Ti4+-doped Li3V2(PO4)3/C fibers for lithium energy storage. Ceram 48(6):8325–8330
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1998) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Ahmadi A, Hadipour NL, Kamfiroozi M, Bagheri Z (2012) Theoretical study of aluminum nitride nanotubes for chemical sensing of formaldehyde. Sens Actuators B Chem 161:1025–1029
Beheshtian J, Kamfiroozi M, Bagheri Z, Ahmadi A (2012) Theoretical study of hydrogen adsorption on the B12P12 fullerene-like nanocluster. Comput Mater Sci 54:115–118
Eid KM, Ammr HY (2011) Adsorption of SO2 on Li atoms deposited on MgO (1 0 0) surface: DFT calculations. Appl Surf Sci 257:6049–6058
Dinadayalane TC, Murray JS, Concha MC, Politzer P, Leszczynski J (2010) Reactivities of sites on (5, 5) single-walled carbon nanotubes with and without a Stone-Wales defect. J Chem Theory Comput 6:1351–1357
Zhao TH, Castillo O, Jahanshahi H, Yusuf A, Alassafi MO, Alsaadi FE, Chu YM (2021) A fuzzy-based strategy to suppress the novel coronavirus (2019-NCOV) massive outbreak. Appl Comput Math 20(1):160–176
Zhao TH, Wang MK, Hai GJ, Chu YM (2022) Landen inequalities for Gaussian hypergeometric function. Revista de la Real Academia de Ciencias Exactas, Físicas y Naturales. Serie A. Matemáticas 116(1):1–23
Nazeer M, Hussain F, Ijaz Khan M, Asad-ur-Rehman E-Z, Chu YM, Malik MY (2021) Theoretical study of MHD electro-osmotically flow of third-grade fluid in micro channel. Appl Math Comput 420:126868
Zhao TH, Khan MI, Chu YM (2021) Artificial neural networking (ANN) analysis for heat and entropy generation in flow of non-Newtonian fluid between two rotating disks. Math Methods Appl Sci
Zhao TH, Wang MK, Zhang W (2018) Chu YM (2018) Quadratic transformation inequalities for Gaussian hypergeometric function. J Inequal Appl 1:251–266
Chu YM, Nazir U, Sohail M, Selim MM, Lee JR (2021) Enhancement in thermal energy and solute particles using hybrid nanoparticles by engaging activation energy and chemical reaction over a parabolic surface via finite element approach. Fractal Fract 5(3):119–136
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Bader RFW (1990) Atoms in molecules: a quantum theory. Clarendon, Oxford
BieglerKönig F, Schönbohm J (2002) Update of the AIM2000-program for atoms in molecules. J Comput Chem 23:1489–1494
Foster JP, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102:7211–7218
Paul BK, Mahanta S, Singh RB, Guchhait N (2010) A DFT-based theoretical study on the photophysics of 4-hydroxyacridine: single-water-mediated excited state proton transfer. J Phys Chem A 114:2618–2627
O’Boyle N, Tenderholt A, Langner K (2008) cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845
Pearson RG (1997) Chemical Hardness. Wiley-VCH, Oxford
Sen KD, Jorgensen CK (1987) Electronegativity, structure and bonding. Springer-Verlag, New York
Li S (2006) Semiconductor physical electronics, 2nd edn. Springer, USA
Bader RFW (1998) A bond path: a universal indicator of bonded interactions. J Phys Chem A 102:7314–7323
Pacios LF (2004) Topological descriptors of the electron density and the electron localization function in hydrogen bond dimers at short intermonomer distances. J Phys Chem A 108:1177–1188
Jenkins S, Morrison I (2000) The chemical character of the intermolecular bonds of seven phases of ice as revealed by ab initio calculation of electron densities. Chem Phys Lett 317:97–102
Arnold WD, Oldfield E (2000) The chemical nature of hydrogen bonding in proteins via NMR: J-couplings, chemical shifts, and AIM theory. J Am Chem Soc 122:12835–12841
Rozas I, Alkorta I, Elguero J (2000) Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. J Am Chem Soc 122:11154–11161
Linares M, Humbel S, Braïda B (2007) Quantifying resonance through a Lewis Valence Bond approach: application to haloallyl and carbonylcations. Faraday Discuss RSC 135:273–283
Salehi N, Vessally E, Edjlali L, Alkorta I, Eshaghi M (2020) Nan@Tetracyanoethylene (n=1-4) systems: sodium salt vs sodium electride. Chem Rev Lett 3:207–217
Sreerama L, Vessally E, Behmagham F (2020) Oxidative lactamization of amino alcohols: an overview. J Chem Lett 1:9–18
Majedi S, Sreerama L, Vessally E, Behmagham F (2020) Metal-free regioselective thiocyanation of (hetero) aromatic C-H bonds using ammonium thiocyanate: an overview. J Chem Lett 1:25–31
Ahmadi R, Kalateh K, Alizadeh R, Khoshtarkib Z, Amani V (2009) Tetra-kis(6-methyl-2,2′-bipyridine)-12 N, N′2 2 N, N′32 N, N′42 N, N′-tetra-nitrato-1:22 O:O′2:33 O:O′, O′′2: 33 O, O′:O′′3:42 O:O′-tetra-nitrato-14 O, O′42 O, O′-tetra-lead(II). Acta Crystallogr E 65:m1169–m1170
Soleimani-Amiri S, Asadbeigi N, Badragheh S (2020) A theoretical approach to new triplet and quintet (nitrenoethynyl) alkylmethylenes, (nitrenoethynyl) alkylsilylenes, (nitrenoethynyl) alkylgermylenes. Iran J Chem Chem Eng (IJCCE) 39(4):39–52
Norouzi N, Ebadi AG, Bozorgian A, Vessally E, Hoseyni SJ (2021) Energy and exergy analysis of internal combustion engine performance of spark ignition for gasoline, methane, and hydrogen fuels. Iran J Chem Chem Eng (IJCCE) 40, in Press
Ma X, Kexin Z, Yonggang W, Ebadi AG, Toughani M (2021) Investigation of low-temperature lipase production and enzymatic properties of Aspergillus Niger. Iran J Chem Chem Eng (IJCCE) 40(4):1364–1374
Vessally E, Mohammadi S, Abdoli M, Hosseinian A, Ojaghloo P (2020) Convenient and robust metal-free synthesis of benzazole-2-ones through the reaction of aniline derivatives and sodium cyanate in aqueous medium. Iran J Chem Chem Eng (IJCCE) 39(5):11–19
Koopmans T (1934) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1:104–113
Sanderson RT (1955) Partial charges on atoms in organic compounds. Science 121:207–208
Sanderson RT (1976) Chemical bonds and bond energy, 2nd edn. Academic Press, New York
Chattaraj PK, Lee H, Parr RG (1991) DFT-based quantitative prediction of regioselectivity: cycloaddition of nitrilimines to methyl propiolate. J Am Chem Soc 113:1855–1856
Gázquez JL (1993) In Chemical hardness. Springer-Verlag, Berlin, Germany
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516
Sridevi C, Shanthi G, Velraj G (2012) Structural, vibrational, electronic, NMR and reactivity analyses of 2-amino-4H-chromene3-carbonitrile (ACC) by ab initio HF and DFT calculations. Spectrochim Acta A Mol Biomol Spectrosc 89:46–54
Raissi H, Khanmohammadi A, Mollania F (2013) A theoretical DFT study on the structural parameters and intramolecular hydrogen-bond strength in substituted (Z)-N-(thionitrosomethylene) thiohydroxylamine systems. Bull Chem Soc Jpn 86:1261–1271
Li X, Deng S, Fu H, Li T (2009) Adsorption and inhibition effect of 6-benzylaminopurine on cold rolled steel in 1.0 M HCl. Electrochim Acta 54:4089–4098
Acknowledgements
The authors wish to thank from Payame Noor University, Tehran, Iran, for their supports.
Author information
Authors and Affiliations
Contributions
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Corresponding author
Ethics declarations
Ethics approval
The manuscript is prepared in compliance with the Ethics in Publishing Policy as described in the Guide for Authors.
Consent to participate
The manuscript is approved by all authors for publication.
Consent for publication
The consent for publication was obtained from all participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hassanpour, A., Poor Heravi, M.R. & Khanmohammadi, A. Electronic sensors for alkali and alkaline earth cations based on Fullerene-C60 and silicon doped on C60 nanocages: a computational study. J Mol Model 28, 148 (2022). https://doi.org/10.1007/s00894-022-05147-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05147-2