Abstract
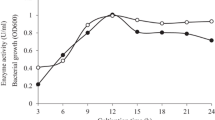
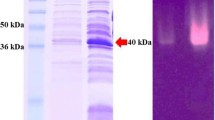
An alkaline β-mannanase was purified to homogeneity from a culture broth of alkaliphilic Bacillus sp. N16-5. The enzyme had optimum activity at pH 9.5 and 70°C. It was composed of a single polypeptide chain with a molecular weight of 55 kDa deduced from SDS-PAGE, and its isoelectric point was around pH 4.3. The enzyme efficiently hydrolyzed galactomannan and glucomannan, producing a series of oligosaccharides and monosaccharides. The β-mannanase gene (manA) contained an open reading frame (ORF) of 1,479 bp, encoding a 32-amino acids signal peptide, and a mature protein of 461 amino acids, with a calculated molecular mass of 50,743 Da. Strain N16-5 ManA, deduced from the manA ORF, exhibited relatively high amino acid similarity to the members of the glycosyl hydrolase family 5. The eight conserved active-site amino acids in family 5 glycosyl hydrolase were found in the deduced amino acid sequence of strain N16-5 ManA.





Similar content being viewed by others
References
Akino T, Nakamura N, Horikoshi K (1987) Production of β-mannosidase and β-mannanase by an alkalophilic Bacillus sp. Appl Microbiol Biotech 26:323–327
Akino T, Nakamura N, Horikoshi K (1988) Characterization of three β-mannanases of an alkalophilic Bacillus sp. Agric Biol Chem 52:773–779
Akino T, Kato C, Horikoshi K (1989) The cloned β-mannanase gene from alkalophilic Bacillus sp. AM-001 produces two β-mannanases in Escherichia coli. Arch Microbiol 152:10–15
Arcand N, Kluepfel D, Paradis FW, Morosoli R, Shareck F (1993) Beta-mannanase of Streptomyces lividans 66: cloning and DNA sequence of the manA gene and characterization of the enzyme. Biochem J 290:857–863
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Bettiol JLP, Boutique JP, Gualco LMP, Johnston JP (2000) Non-aqueous liquid detergent compositions comprising a borate-releasing compound and a mannanase. EP1059351
Bettiol JP, Showell MS (2002) Detergent compositions comprising a mannanase and a protease. US Patent 6,376,445
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Braithwaite KL, Black GW, Hazlewood GP, Ali BRS, Gilbert HJ (1995) A non-modual endo-β-1,4-mannanase from Pseudomonas fluorescens subspecies cellulose. Biochem J 305:1005–1010
Buchert J, Salminen J, Sika-aho M, Ranua M, Viikari L (1993) The role of Trichodema reesei xylanase and mannanase in the treatment of softwood kraft pulp prior to bleaching. Holzforschung 47:473–478
Duffaud GD, McCutchen CM, Leduc P, Parker KN, Kelly RM (1997) Purification and characterization of extremely thermostable β-mannannase, β-mannasidase, and α-galactosidase from the hyperthermophilic eubacterium Thermotoga neapolitana 5068. Appl Environ Microbiol 63:169–177
Emi S, Fukumoto J, Yamamoto T (1972) Crystallization and some properties of mannanase. Agric Biol Chem 36:991–1001
Ethier N, Talbot G, Sygusch J (1998) Gene cloning, DNA sequencing, and expression of thermostable β-mannanase from Bacillus stearothermophilus. Appl Environ Microbiol 64:4428–4432
Gibbs MD, Elinder AU, Reeves RA, Bergquist PL (1996) Sequencing, cloning and expression of a β-mannannase gene, manA, from the extremely thermophilic anaerobic bacterium, Caldicellulosiruptor RT8B.4. FEMS Microbiol Lett 141:37–43
Henrissat B (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 280:309–316
Hilge M, Gloor SM, Rypniewski W, Sauer O, Heightman TD, Zimmermann W, Winterhalter K, Piontek K (1998) High-resolution native and complex structures of thermostable beta-mannanase from Thermomonospora fusca substrate specificity in glycosyl hydrolase family 5. Structure 6:1433–1444
Horikoshi K (1971) Production of alkaline enzymes by alkalophilic microorganisms. Part I. Alkaline protease produced by Bacillus no. 221. Agric Biol Chem 36:1407–1414
Horikoshi K (1996) Alkaliphiles—from an industrial point of view. FEMS Microbiol Rev 18:259–270
Ito S, Kobayashi T, Ara K, Ozaki K, Kawai S, Hatada Y (1998) Alkaline detergent enzymes from alkaliphiles: enzymatic properties, genetics, and structures. Extremophiles 2:185–190
Kauppinen MS, Schulein M, Schnorr K, Andersen LN, Bjørnvad ME (2003) Mannanases. US Patent 6,566,114
Khanongnuch C, Lumyong S, Ooi T, Kinoshita S (1999) A non-cellulase producing strain of Bacillus subtilis and its potential use in pulp biobleaching. Biotechnol Lett 21:61–63
Kobayashi Y, Echizen R, Mada M, Mutai M (1984) Intestinal flora and dietary factors. In: Mitsuoka T (ed) Proceedings of the 4th RIKEN Symposium on Intestinal flora, Tokyo, 1983. Japan Scientific Societies Press, Tokyo, pp 69–90
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Ma Y, Tian X, Zhou P, Wang D (1991) Production and some properties of alkaline β-mannanase (in Chinese). Acta Microbiol Sin 31:443–448
McCutchen CM, Duffaud GD, Leduc P, Peterson ARH, Tayal A, Khan SA, Kelly RM (1996) Characterization of extremely thermostable enzymatic breakers α-1,6-galactosidase and β-1,4-mannanase) from the hyperthermophilic bacterium Thermotoga neapolitana 5068 for hydrolysis of guar gum. Biotechnol Bioeng 52:332–339
Perlman D, Halvorson HO (1983) A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol 167:391–409
Reese T, Shibata Y (1965) β-mannanase of fungi. Can J Microbiol 11:167–183
Sakon J, Adney WS, Himmel ME, Thomas SR, Karplus PA (1996) Crystal structure of thermostable family 5 endocellulase E1 from Acidothermus cellulolyticus in complex with cellotetraose. Biochemistry 35:10648–10660
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Shimahara H, Suzuki H, Sugiyama N, Nisizawa K (1975) Partial purification of β-mannanase from the konjac tubers and their substrate specificity in relation to the structure of konjac glucomannan. Agric Biol Chem 39:301–312
Teather RM, Wood PJ (1982) Use of Congo Red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43:777–780
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignments through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680
Welker NE, Campbell LL (1963) Effect of carbon source on formation of α-amylase by Bacillus stearothermophilus. J Bacteriol 86:681–686
Zhang W, Ma Y, Xue Y, Zhou P, Ventosa A, Grant WD (2002) Salinicoccus alcaliphilus sp. nov., a new alkaliphilic and moderately halophilic Gram-positive coccus. Int J Syst Evol Microbiol 52:789–793
Acknowledgements
We thank Prof. W.D. Grant for his kind advice. This work was supported by the Chinese Academy of Sciences, by the Ministry of Science and Technology of China, and by the National Basic Research Program of China (no. 2003CB716001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Horikoshi
Rights and permissions
About this article
Cite this article
Ma, Y., Xue, Y., Dou, Y. et al. Characterization and gene cloning of a novel β-mannanase from alkaliphilic Bacillus sp. N16-5. Extremophiles 8, 447–454 (2004). https://doi.org/10.1007/s00792-004-0405-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-004-0405-4