Abstract
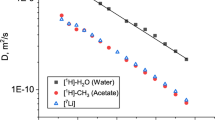
The development of ecofriendly electrolytes for lithium-ion batteries is one of the actual tasks of modern electrochemistry. In particular, to this purpose, the highly concentrated ternary aqueous systems based on lithium acetate (LiOAc) have been actively investigated. Here, the diffusion coefficients of 7Li+ and 133Cs+ cations, OAc– anion, as well as water (1H), in ternary aqueous solutions of cesium and lithium acetates in a range of temperature (– 15 ÷ 35 °C) have been measured using the PFG NMR method. A direct attempt to interpret the obtained dependences within the framework of the Stokes–Einstein model led to the fact that the calculated hydrodynamic radius of the Cs+ cation turned out to be noticeably smaller than its crystallographic one. An approach to describing the high rate of diffusion of cesium cations is proposed, based on taking into account the local viscosity near cations of both types. The use of the approach allowed us to calculate more correctly the hydrodynamic radii of cations, while remaining within the framework of the Stokes–Einstein model. As a result, it has been possible to describe the features of translational motion of components in a complex system that is interesting for electrochemical applications.





Similar content being viewed by others
Availability of Data and Materials
Not applicable to this study.
References
S. Chen, M. Zhang, P. Zou, B. Sun, S. Tao, Historical development and novel concepts on electrolytes for aqueous rechargeable batteries. Energy Environ. Sci. 15(5), 1805–1839 (2022). https://doi.org/10.1039/D2EE00004K
S.K. Sharma et al., Progress in electrode and electrolyte materials: path to all-solid-state Li-ion batteries. Energy Adv. 8(1), 457–510 (2022). https://doi.org/10.1039/D2YA00043A
M.R. Lukatskaya et al., Concentrated mixed cation acetate “water-in-salt” solutions as green and low-cost high voltage electrolytes for aqueous batteries. Energy Environ. Sci. 11(10), 2876–2883 (2018). https://doi.org/10.1039/C8EE00833G
V.V. Matveev, O.N. Pestova, K.V. Tyutyukin, V.I. Chizhik, Molecular mobility in mixed “water-in-salt” solutions of LiOAc and KOAc according to NMR data. Appl. Magn. Reson. 54(10), 971–978 (2023). https://doi.org/10.1007/s00723-023-01558-3
S. Chen, R. Lan, J. Humphreys, S. Tao, Effect of cation size on alkali acetate-based ‘water-in-bisalt’ electrolyte and its application in aqueous rechargeable lithium battery. Appl. Mater. Today 20, 100728 (2020). https://doi.org/10.1016/j.apmt.2020.100728
S. Liu et al., Caesium acetate-based electrolytes for aqueous electrical double layer capacitors. ChemElectroChem 9(22), e20220071 (2022). https://doi.org/10.1002/celc.202200711
L. Liangbin et al., Patent CN, no. CN116283548A, 2023.
V.I. Chizhik, Magnetic Resonance and Its Applications (Springer, Cham, 2014)
M.H. Levitt, Spin Dynamics: Basics of Nuclear Magnetic Resonance, 2nd edn. (Wiley, Chichester, 2008)
D. Burstein, Stimulated echoes: description, applications, practical hints. Concepts Magn. Reson.Magn. Reson. 8(4), 269–278 (1996). https://doi.org/10.1002/(SICI)1099-0534(1996)8:4%3c269::AID-CMR3%3e3.0.CO;2-X
E.O. Stejskal, J.E. Tanner, Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J. Chem. Phys. 42(1), 288–292 (1965). https://doi.org/10.1063/1.1695690
G.H. Sorland, Dynamic Pulsed-Field-Gradient. NMR Springer Series in Chemical Physics (Springer, Berlin, 2014), p.110
K. Hayamizu, Yu. Chiba, T. Haishi, Dynamic ionic radius of alkali metal ions in aqueous solution: a pulsed-field gradient NMR study. RSC Adv. 11, 20252 (2021). https://doi.org/10.1039/D1RA02301B
K. Hayamizu, S. Tsuzuki, S. Seki, Y. Umebayashi, Nuclear magnetic resonance studies on the rotational and translational motions of ionic liquids composed of 1-ethyl-3-methylimidazolium cation and bis(trifluoromethanesulfonyl)amide and bis(fluorosulfonyl)amide anions and their binary systems including lithium salts. J. Chem. Phys. 135, 084505 (2011). https://doi.org/10.1063/1.3625923
V.I. Chizhik, NMR relaxation and microstructure of aqueous electrolyte solutions. Mol. Phys. 90, 653–659 (1997). https://doi.org/10.1080/00268979709482647
J.D. Bernal, R.H. Fowler, A theory of water and ionic solution, with particular reference to hydrogen and hydroxyl ions. J. Chem. Phys. 1(8), 515–548 (1933). https://doi.org/10.1063/1.1749327
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Found. Crystallogr. 32, 751–767 (1976). https://doi.org/10.1107/S0567739476001551
Acknowledgements
NMR measurements were carrying out in the Center for Magnetic Resonance of Research Park of St. Petersburg State University.
Funding
This work was supported by the Russian Science Foundation (Project no. 23-23-00049).
Author information
Authors and Affiliations
Contributions
K.A.M. prepared samples, conducted experimental measurements and processing results, prepared all figures; O.N.P. synthesized anhydrous cesium acetate, participated in the explanation of the results; V.V.M. analyzed the literature data, interpreted the results and participated in preparing the article text; V.I.C. analyzed the experimental results obtained, developed an approach for interpreting the results and participated in preparing the article text.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mukhin, K.A., Pestova, O.N., Matveev, V.V. et al. Translational Mobility in Ternary Systems “Lithium Acetate–Cesium Acetate–Water” According to PFG NMR Data. Appl Magn Reson (2024). https://doi.org/10.1007/s00723-024-01670-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00723-024-01670-y