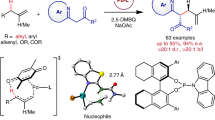
Abstract
Rh-catalyzed tandem hydroformylation/isomerization/hydrogenation of various 1-arylbutadienes toward the corresponding branched saturated aldehydes were performed in the presence of monodentate phosphine ligand PPh3. After investigating different reaction parameters such as P-ligands and solvents, the conversion of substrates (including electron withdrawing and electron donating groups on aromatic rings, up to 99%) and chemical/regioselectivity of the target products (up to 98% and 99/1 for B/L) were improved using DMF as solvent. The [Rh(cod)Cl]2/PPh3 catalytic system had good substrate universality in terms of substituents on the phenyl ring and provided a simple synthesis method for branched saturated aldehydes.
Graphical abstract

Similar content being viewed by others
Data availability
The data that support the findings of this study are available in Supporting Information, which associated with the experiments can be found in the online version.
References
Franke R, Selent D, Börner A (2012) Chem Rev 112:5675
Pospech J, Fleischer I, Franke R, Buchholz S, Beller M (2013) Angew Chem Int Ed 52:2852
Cai CX, Yu SC, Liu GD, Zhang XW, Zhang XM (2011) Adv Synth Catal 353:2665
Wang P, Shi HB, Feng BL, Zhao DM, Wu HG (2023) Monatsh Chem 154:1189
Guo XH, Shen JX (2014) Monatsh Chem 145:657
Breit B (2003) Acc Chem Res 36:264
Gaide T, Bianga J, Schlipköter J, Behr J, Vorholt AJ (2017) ACS Catal 7:4163
Mao ZT, **e ZH, Chen JG (2021) ACS Catal 11:14575
Zhou W, He DH (2009) Catal Lett 127:437
Wang P, Liu H, Yang D (2021) Chem J Chin Univ 42:3024
Watkins AL, Landis CR (2011) Org Lett 13:164
Horiuchi T, Ohta T, Shirakawa E, Nozaki K, Takaya H (1997) Tetrahedron 53:7795
Vieira GM, Granato AV, Gusevskaya EV, Santos EN, Dixneuf PH, Fischmeister C, Bruneau C (2020) Appl Catal A Gen 598:117583
Hoffmann RW, Rohde T, Haeberlin E, Schäfer F (1999) Org Lett 1:1713
Hoffmann RW, Haeberlin E, Rohde T (2002) Synthesis 2:207
Aikawa K, Ishii K, Endo Y, Mikami K (2017) J Fluorine Chem 203:122
Vyas DJ, Larionov E, Besnard C, Guénée L, Mazet C (2013) J Am Chem Soc 135:6177
Humbert N, Vyas DJ, Besnard C, Mazet C (2014) Chem Commun 50:10592
Yan X, Qiao CH, Guo ZW (2013) Synlett 24:502
Noonan GM, Fuentes JA, Cobley CJ, Clarke ML (2012) Angew Chem Int Ed 51:2477
Gaspar B, Carreira EM (2009) J Am Chem Soc 131:13214
Chatani N, Fujii S, Yamasaki Y, Murai S, Sonoda N (1986) J Am Chem Soc 108:7361
Wu FJ, Wang LF, Chen J, Nicewicz DA, Huang Y (2018) Angew Chem Int Ed 57:2174
Nagao K, Yokobori U, Makida Y, Ohmiya H, Sawamura M (2012) J Am Chem Soc 134:898
Liu J, Dang HS (2012) J Chem Res 36:732
Cabré A, Giménez JC, Sciortino G, Ujaque G, Verdaguer X, Lledós A, Riera A (2019) Adv Synth Catal 361:1
Neubert P, Fuchs S, Behr A (2015) Green Chem 17:4045
Behr A, Reyer S, Tenhumberg N (2011) Dalton Trans 40:11742
Fell B, Bahrmann H (1980) J Mol Catal 8:329
Wang P, Yang D, Liu H (2021) Chin J Org Chem 41:3379
Klosin J, Landis CR (2007) Acc Chem Res 40:1251
Leeuwen PWNMV, Roobeek CF (1985) J Mol Catal 31:2211
Liu G, Garland M (2000) J Organ Chem 608:76
Smith SE, Rosendahl T, Hofmann P (2011) Organometallics 30:3643
Abkai G, Schmidt S, Rosendahl T, Rominger F, Hofmann P (2014) Organometallics 33:3212
Horiuchi T, Ohta T, Nozaki K, Takaya H (1996) Chem Commun 2:155
Wong GW, Landis CR (2013) Angew Chem Int Ed 52:1564
Adint TT, Wong GW, Landis CR (2013) J Org Chem 78:4231
Saha B, Rajan Babu TV (2006) Org Lett 8:4657
Wang P, Liu H, Li YQ, Zhao XL, Lu Y, Liu Y (2016) Catal Sci Technol 6:3854
Wang P, Wang DL, Liu H, Zhao XL, Lu Y, Liu Y (2017) Organometallics 36:2404
Wang P, Liu L, Luo ZJ, Zhou Q, Lu Y, **a F, Liu Y (2018) J Catal 361:230
Wang P, Liu H, Yang D (2022) Prog Chem 34:1076
Zhao JG, Yi JW, Yang CJ, Wan KF, Duan XX, Tang SB, Fu HY, Zheng XL, Yuan ML, Li RX, Chen H (2021) Catal Lett 151:1273
Li X, Qin TT, Li LS, Wu B, Lin TJ, Zhong LS (2021) Catal Lett 151:2638
Arai H (1978) J Catal 51:135
Bungu PN, Otto S (2007) Dalton Trans 27:2876
Chauvin Y, Mussmann L, Olivier H (1996) Angew Chem Int Ed 34:2698
Winkle JL, Morris RC, Mason RF (1969) Single stage hydroformylation of olefins to alcohols. US patent 3420898, Jan 07, 1969; (1967) Chem Abstr 66:65101
Macaluso A, Rigdon OW (1975) Synthesis of linear primary alcohols from internal olefins. US patent 3907909, Sept 23, 1975; (1977) Chem Abstr 86:43169
Mul WP (2007) Hydroformylation process for production of alcohols and aldehydes. Patent WO2007003589, Jan 11, 2007; (2007) Chem Abstr 146:144687
Kieffer EP, Renkema D, Sietsma JRA (2013) A process for the preparation of detergent compounds with a relatively low amount of isoparaffins, wherein the detergents are derived from a Fischer–Tropsch product stream. European patent EP 2610325, July 2, 2013; (2013) Chem Abstr 159:182948
De Boer-Wildschut M, Charernsuk M, Krom CA, Pringle PG (2012) Ligand, catalyst and process for hydroformylation. Patent WO 2012072594, June 7, 2012; (2012) Chem Abstr 157:45347
Yuki Y, Takahashi K, Tanaka Y, Nozak K (2013) J Am Chem Soc 135:17393
Wu LP, Fleischer I, Jackstell R, Profir I, Franke R, Beller M (2013) J Am Chem Soc 135:14306
Yu SM, Snavely WK, Chaudhari RV, Subramaniam B (2020) Mol Catal 484:110721
Polo A, Claver C, Castillbn S, Ruiz A (1992) Organometallics 11:3525
Baybn JC, Real J, Claver C, Polo A, Ruiz A (1989) J Chem Soc Chem Commun 15:1056
Ding ST, Jiao N (2012) Angew Chem Int Ed 51:9226
Petersen TP, Larsen AF, Ritzen A, Ulven T (2013) J Org Chem 78:4190
Muzart J (2009) Tetrahedron 65:8313
Qian JQ, Zhu HX, Shi BB, Huang A, Gou LH (2023) J Chem Technol Biotechnol 98:381
Wolf EY, Vartanyan MM, Lapidus AL (2013) Petrol Chem 53:194
Rösler T, Ehmann KR, Köhnke K, Leutzsch M, Wessel N, Vorholt AJ, Leitner W (2021) J Catal 400:234
Leeuwen PWNMV, Sandee AJ, Reek JNH, Kamer PCJ (2002) J Mol Catal A Chem 182–183:107
Leeuwen PWNMV, Roobeek CF (1985) J Mol Catal 31:345
Foca CM, Barros HJV, Santos EN, Gusevskaya EV, Bayon JC (2003) New J Chem 27:533
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21901250).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, H., Wang, P., Yan, Y. et al. Highly regioselective synthesis of branched saturated aldehydes by tandem hydroformylation/isomerization/hydrogenation of 1-arylbutadienes. Monatsh Chem 155, 731–738 (2024). https://doi.org/10.1007/s00706-024-03224-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-024-03224-1