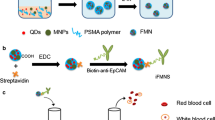
Abstract
The enumeration of circulating tumor cells (CTCs) in peripheral blood plays a crucial role in the early diagnosis, recurrence monitoring, and prognosis assessment of cancer patients. There is a compelling need to develop an efficient technique for the capture and identification of these rare CTCs. However, the exclusive reliance on a single criterion, such as the epithelial cell adhesion molecule (EpCAM) antibody or aptamer, for the specific recognition of epithelial CTCs is not universally suitable for clinical applications, as it usually falls short in identifying EpCAM-negative CTCs. To address this limitation, we propose a straightforward and cost-effective method involving triplex fluorescently labelled aptamers (FAM-EpCAM, Cy5-PTK7, and Texas Red-CSV) to modify Fe3O4-loaded dendritic SiO2 nanocomposite (dmSiO2@Fe3O4/Apt). This multi-recognition-based strategy not only enhanced the efficiency in capturing heterogeneous CTCs, but also facilitated the rapid and accurate identification of CTCs. The capture efficiency of heterogenous CTCs reached up to 93.33%, with a detection limit as low as 5 cells/mL. Notably, the developed dmSiO2@Fe3O4/Apt nanoprobe enabled the swift identification of captured cells in just 30 min, relying solely on the fluorescently modified aptamers, which reduced the identification time by approximately 90% compared with the conventional immunocytochemistry (ICC) technique. Finally, these nanoprobe characteristics were validated using blood samples from patients with various types of cancers.
Graphical Abstract







Similar content being viewed by others
Data availability
Data will be made available on request.
References
Diamantopoulou Z, Castro-Giner F, Schwab FD, Foerster C, Saini M, Budinjas S, Strittmatter K, Krol I, Seifert B, Heinzelmann-Schwarz V, Kurzeder C, Rochlitz C, Vetter M, Weber WP, Aceto N (2022) The metastatic spread of breast cancer accelerates during sleep. Nature 607:156–162. https://doi.org/10.1038/s41586-022-04875-y
Kwizera EA, Ou W, Lee S, Stewart S, Shamul JG, Xu J, Tait N, Tkaczuk KHR, He X (2022) Correction to greatly enhanced CTC culture enabled by capturing CTC heterogeneity using a PEGylated PDMS-titanium-gold electromicrofluidic device with glutathione-controlled gentle cell release. ACS Nano 16:19606. https://doi.org/10.1021/acsnano.2c10382
Ma J, Chen Y, Ren J, Zhou T, Wang Z, Li C, Qiu L, Gao T, Ding P, Ding Z, Ou L, Wang J, Xu J, Zhou Z, Jia C, Sun N, Pei R, Zhu W (2023) Purification of circulating tumor cells based on multiantibody-modified magnetic nanoparticles and molecular analysis toward epithelial ovarian cancer detection. ACS Sens 8:3744–3753. https://doi.org/10.1021/acssensors.3c01063
Wu Y, Zhou Y, Paul R, Qin X, Islam K, Liu Y (2022) Adaptable microfluidic vessel-on-a-chip platform for investigating tumor metastatic transport in bloodstream. Anal Chem 94:12159–12166. https://doi.org/10.1021/acs.analchem.2c02556
Long Y, Lu Z, Xu S, Li M, Wang X, Zhang Z, He Q (2020) Self-delivery micellar nanoparticles prevent premetastatic niche formation by interfering with the early recruitment and vascular destruction of granulocytic myeloid-derived suppressor cells. Nano Lett 20:2219–2229. https://doi.org/10.1021/acs.nanolett.9b03883
Chowdhury T, Cressiot B, Parisi C, Smolyakov G, Thiébot B, Trichet L, Fernandes FM, Pelta J, Manivet P (2023) Circulating tumor cells in cancer diagnostics and prognostics by single-molecule and single-cell characterization. ACS Sens 8:406–426. https://doi.org/10.1021/acssensors.2c02308
Lv S, Zheng D, Chen Z, Jia B, Zhang P, Yan J, Jiang W, Zhao X, Xu JJ (2023) Near-infrared light-responsive size-selective lateral flow chip for single-cell manipulation of circulating tumor cells. Anal Chem 95:1201–1209. https://doi.org/10.1021/acs.analchem.2c03947
Wang D, Dong R, Wang X, Jiang X (2022) Flexible electronic catheter based on nanofibers for the in vivo elimination of circulating tumor cells. ACS Nano 16:5274–5283. https://doi.org/10.1021/acsnano.1c09807
Cheng SB, Chen MM, Wang YK, Sun ZH, Qin Y, Tian S, Dong WG, **e M, Huang WH (2021) A three-dimensional conductive scaffold microchip for effective capture and recovery of circulating tumor cells with high purity. Anal Chem 93:7102–7109. https://doi.org/10.1021/acs.analchem.1c00785
Li C, Li R, Wu X, Zuo Y, **ong G, Huang M, Sun Y, Liao R, **ao Y, Hu L, Gao C, Yu Y (2022) Capture of heterogeneous circulating tumor cells in Colorectal Cancer patients on an immunomagnetic and anti-nonspecific adsorption platform. Anal Chem 94:15240–15249. https://doi.org/10.1021/acs.analchem.2c02416
Zhu L, Feng X, Yang S, Wang J, Pan Y, Ding J, Li C, Yin X, Yu Y (2021) Colorimetric detection of immunomagnetically captured rare number CTCs using mDNA-wrapped single-walled carbon nanotubes. Biosens Bioelectron 172:112780. https://doi.org/10.1016/j.bios.2020.112780
Chen P, Wang Y, He Y, Huang K, Wang X, Zhou R, Liu T, Qu R, Zhou J, Peng W, Li M, Bai Y, Chen J, Huang J, Geng J, **e Y, Hu W, Ying B (2021) Homogeneous visual and fluorescence detection of circulating tumor cells in clinical samples via selective recognition reaction and enzyme-free amplification. ACS Nano 15:11634–11643. https://doi.org/10.1021/acsnano.1c02080
**a W, Li H, Li Y, Li M, Fan J, Sun W, Li N, Li R, Shao K, Peng X (2021) In vivo coinstantaneous identification of hepatocellular carcinoma circulating tumor cells by dual-targeting magnetic-fluorescent nanobeads. Nano Lett 21:634–641. https://doi.org/10.1021/acs.nanolett.0c04180
Dou R, Liu K, Yang C, Zheng J, Shi D, Lin X, Wei C, Zhang C, Fang Y, Huang S, Song J, Wang S, **ong B (2021) EMT-cancer cells-derived exosomal miR-27b-3p promotes circulating tumour cells-mediated metastasis by modulating vascular permeability in colorectal cancer. Clin Transl Med 11:e595. https://doi.org/10.1002/ctm2.595
Zhang Q, Rong Y, Yi K, Huang L, Chen M, Wang F (2020) Circulating tumor cells in hepatocellular carcinoma: single-cell based analysis, preclinical models, and clinical applications. Theranostics 10:12060–12071. https://doi.org/10.7150/thno.48918
Zhang Z, Wuethrich A, Wang J, Korbie D, Lin LL, Trau M (2021) Dynamic monitoring of EMT in CTCs as an indicator of cancer metastasis. Anal Chem 93:16787–16795. https://doi.org/10.1021/acs.analchem.1c03167
Visal TH, den Hollander P, Cristofanilli M, Mani SA (2022) Circulating tumour cells in the -omics era: how far are we from achieving the ‘singularity’? Br J Cancer 127:173–184. https://doi.org/10.1038/s41416-022-01768-9
Myung JH, Cha A, Tam KA, Poellmann M, Borgeat A, Sharifi R, Molokie RE, Votta-Velis G, Hong S (2019) Dendrimer-based platform for Effective capture of Tumor cells after TGFβ(1)-Induced epithelial-mesenchymal transition. Anal Chem 91:8374–8382. https://doi.org/10.1021/acs.analchem.9b01181
Li C, Yang S, Li R, Gong S, Huang M, Sun Y, **ong G, Wu D, Ji M, Chen Y, Gao C, Yu Y (2022) Dual-aptamer-targeted immunomagnetic nanoparticles to accurately explore the correlations between circulating Tumor cells and gastric Cancer. ACS Appl Mater Interfaces 14:7646–7658. https://doi.org/10.1021/acsami.1c22720
Zuo Y, **a Y, Lu W, Li Y, **ao Y, Gao S, Zhou Z, Xu H, Feng X, Li C, Yu Y (2023) A multifunctional black phosphorus nanosheet-based immunomagnetic bio-interface for heterogeneous circulating tumor cell capture and simultaneous self-identification in gastric cancer patients. Nanoscale 15:3872–3883. https://doi.org/10.1039/d2nr04277k
Zheng Y, Zhang J, Huang M, Wang T, Qu X, Wu L, Song J, Wang W, Song Y, Yang C (2020) Selection of aptamers against vimentin for isolation and release of circulating tumor cells undergoing epithelial mesenchymal transition. Anal Chem 92:5178–5184. https://doi.org/10.1021/acs.analchem.9b05690
Zuo Y, Lu W, **a Y, Meng J, Zhou Y, **ao Y, Zhu L, Liu D, Yang S, Sun Y, Li C, Yu Y (2023) Glucometer readout for portable detection of heterogeneous circulating tumor cells in lung cancer captured on a dual aptamer functionalized wrinkled cellulose hydrogel interface. ACS Sens 8:187–196. https://doi.org/10.1021/acssensors.2c02029
Yu Y, Yang Y, Ding J, Meng S, Li C, Yin X (2018) Design of a biocompatible and ratiometric fluorescent probe for the capture, detection, release, and reculture of rare number CTCs. Anal Chem 90:13290–13298. https://doi.org/10.1021/acs.analchem.8b02625
Wideman NE, Oliver JD, Crandall PG, Jarvis NA (2021) Detection and potential virulence of viable but non-culturable (VBNC) Listeria monocytogenes: a review. Microorganisms 9:194. https://doi.org/10.3390/microorganisms9010194
Zhang Q, Wu M, Fang Y, Deng C, Shen HH, Tang Y, Wang Y (2022) Dendritic mesoporous silica hollow spheres for nano-bioreactor application. Nanomaterials (Basel) 12:1940. https://doi.org/10.3390/nano12111940
Hernández P, Lucero-Acuña A, Moreno-Cortez IE, Esquivel R, Álvarez-Ramos E (2020) Thermo-magnetic properties of Fe₃O₄@Poly(N-Isopropylacrylamide) Core-Shell nanoparticles and their cytotoxic effects on HeLa and MDA-MB-231 cell lines. J Nanosci Nanotechnol 20:2063–2071. https://doi.org/10.1166/jnn.2020.17324
Wei Z, Ma X, Zhang Y, Guo Y, Wang W, Jiang ZY (2022) High-efficiency adsorption of phenanthrene by Fe(3)O(4)-SiO(2)-dimethoxydiphenylsilane nanocomposite: experimental and theoretical study. J Hazard Mater 422:126948. https://doi.org/10.1016/j.jhazmat.2021.126948
Cai W, Guo M, Weng X, Zhang W, Owens G, Chen Z (2020) Modified green synthesis of Fe(3)O(4)@SiO(2) nanoparticles for pH responsive drug release. Mater Sci Eng C Mater Biol Appl 112:110900. https://doi.org/10.1016/j.msec.2020.110900
Stachurska X, Cendrowski K, Pachnowska K, Piegat A, Mijowska E, Nawrotek P (2022) Nanoparticles influence lytic phage T4-like performance in Vitro. Int J Mol Sci 23:7179. https://doi.org/10.3390/ijms23137179
Funding
We appreciated the financial supports from Jiangsu Provincial Key Laboratory of New Drug Research and Clinical Pharmacy Open Research Project (KFKT-2304) and Young Innovative Talent Funding Project (QC202216).
Author information
Authors and Affiliations
Contributions
**nwen Wang: investigation, data curation, writing-original draft. Yu Du: methodology. Weijun **g: visualization, formal analysis. Changchun Cao: TEM, HRTEM, SEM, XRD, VSM, Zeta tests. **aoli Wu: clinical blood sample collections. Kangqun Yang: validation and red blood cell lysis. Liang Zhu: conceptualization, funding acquisition, writing-review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study received approval from the Committee on Ethics at the Affiliated Huaian No.1 People’s Hospital of Nan**g Medical University (approval number: YX-Z-2023-034-01), and all participants provided informed consent prior to their participation.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Du, Y., **g, W. et al. Fluorescent identification of immunomagnetically captured CTCs using triplex-aptamer-targeted dendritic SiO2@Fe3O4 nanocomposite. Microchim Acta 191, 424 (2024). https://doi.org/10.1007/s00604-024-06504-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06504-z