Abstract
A flexible, wearable, non-invasive contact lens sensor utilizing nickel-cobalt metal-organic framework (Ni-Co-MOF) based hydrogel is introduced for urea monitoring in tear samples. The synthesized Ni-Co-MOF hydrogel exhibits a porous structure with interconnected voids, as visualized by Scanning Electron Microscopy (SEM). Detailed structural and vibrational properties of the material were characterized using X-ray Diffraction (XRD), Fourier Transform Infrared (FTIR) spectroscopy, and Raman spectroscopy. The developed Ni-Co-MOF hydrogel sensor showcases a detection limit of 0.445 mM for urea within a linear range of 0.5–70 mM. Notably, it demonstrates exceptional selectivity, effectively distinguishing against interfering species like UA, AA, glucose, dopamine, Cl−, K+, Na+, Ca2+, and IgG. The enhanced electrocatalytic performance of the Ni-Co-MOF hydrogel electrode is attributed to the presence of Ni and Co, fostering Ni2+ oxidation on the surface and forming a Co2+ complex that acts as a catalyst for urea oxidation. The fabricated sensor exhibits successful detection and retrieval of urea in simulated tear samples, showcasing promising potential for bioanalytical applications. The binder-free, non-toxic nature of the Ni-Co-MOF hydrogel sensor presents exciting avenues for future utilization in non-enzymatic electrochemical sensing, including applications in wearable devices, point-of-care diagnostics, and personalized healthcare monitoring.
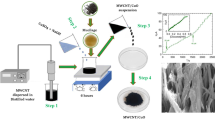
Graphical Abstract







Similar content being viewed by others
References
Gerard M, Chaubey A, Malhotra BD (2002) Application of conducting polymers to biosensors. Biosens Bioelectron 17(5):345–359. https://doi.org/10.1016/S0956-5663(01)00312-8
Dhand C, Das M, Datta M, Malhotra BD (2011) Recent advances in polyaniline based biosensors. Biosens Bioelectron 26(6):2811–2821. https://doi.org/10.1016/j.bios.2010.10.017
Yang X et al (2020) Flexible, wearable microfluidic contact lens with capillary networks for tear diagnostics. J Mater Sci 55(22):9551–9561. https://doi.org/10.1007/S10853-020-04688-2/METRICS
Mishra GK, Mishra RK, Bhand S (2010) Flow injection analysis biosensor for urea analysis in adulterated milk using enzyme thermistor. Biosens Bioelectron 26(4):1560–1564. https://doi.org/10.1016/j.bios.2010.07.113
Kukkar D, Zhang D, Jeon BH, Kim K-H (2022) Recent advances in wearable biosensors for non-invasive monitoring of specific metabolites and electrolytes associated with chronic kidney disease: performance evaluation and future challenges. TrAC Trends Anal Chem 150:116570. https://doi.org/10.1016/j.trac.2022.116570
Remiszewska E et al (2019) Enzymatic method of urea determination in LTCC microfluidic system based on absorption photometry. Sensors Actuators B Chem 285:375–384. https://doi.org/10.1016/j.snb.2019.01.032
Ali N, Ismail M, Khan A, Khan H, Haider S, Kamal T (2018) Spectrophotometric methods for the determination of urea in real samples using silver nanoparticles by standard addition and 2nd order derivative methods. Spectrochim Acta Part A Mol Biomol Spectrosc 189:110–115. https://doi.org/10.1016/j.saa.2017.07.063
Ismail M et al (2022) Role of silver nanoparticles in fluorimetric determination of urea in urine samples. Spectrochim Acta Part A Mol Biomol Spectrosc 271:120889. https://doi.org/10.1016/j.saa.2022.120889
Koebel M, Elsener M (1995) Determination of urea and its thermal decomposition products by high-performance liquid chromatography. J Chromatogr A 689(1):164–169. https://doi.org/10.1016/0021-9673(94)00922-V
Liu L, Mo H, Wei S, Raftery D (2012) Quantitative analysis of urea in human urine and serum by 1 H nuclear magnetic resonance. Analyst 137(3):595–600. https://doi.org/10.1039/C2AN15780B
da Costa Filho PA, Cobuccio L, Mainali D, Rault M, Cavin C (2020) Rapid analysis of food raw materials adulteration using laser direct infrared spectroscopy and imaging. Food Control 113:107114. https://doi.org/10.1016/j.foodcont.2020.107114
Elmasry MR et al (2023) Fluorometric and colorimetric hybrid carbon-dot nanosensors for dual monitoring of urea. ACS Appl Nano Mater 6(9):7992–8003. https://doi.org/10.1021/ACSANM.3C01247/ASSET/IMAGES/LARGE/AN3C01247_0006.JPEG
Simeral LS (1997) Determination of urea, nitrate, and ammonium in aqueous solution using nitrogen-14 nuclear magnatic resonance. Appl Spectrosc 51(10):1585–1587. https://doi.org/10.1366/0003702971939145/ASSET/0003702971939145.FP.PNG_V03
Roch-Ramel F (1967) An enzymic and fluorophotometric method for estimating urea concentrations in nanoliter specimens. Anal Biochem 21(3):372–381. https://doi.org/10.1016/0003-2697(67)90312-0
Hu L et al (2023) Hydrogel-based flexible electronics. Adv Mater 35(14):2205326. https://doi.org/10.1002/adma.202205326
Mondal S, Sangaranarayanan MV (2013) A novel non-enzymatic sensor for urea using a polypyrrole-coated platinum electrode. Sensors Actuators B Chem 177:478–486. https://doi.org/10.1016/j.snb.2012.11.031
Ezhilan M et al (2017) Design and development of electrochemical biosensor for the simultaneous detection of melamine and urea in adulterated milk samples. Sensors Actuators B Chem 238:1283–1292. https://doi.org/10.1016/j.snb.2016.09.100
Zyoud SH et al (2023) Structural, optical, and electrical investigations of Nd2O3-doped PVA/PVP polymeric composites for electronic and optoelectronic applications. Polymers (Basel) 15(6):1351. https://doi.org/10.3390/polym15061351
Liao M, Liao H, Ye J, Wan P, Zhang L (2019) Polyvinyl alcohol-stabilized liquid metal hydrogel for wearable transient epidermal sensors. ACS Appl Mater Interfaces 11(50):47358–47364. https://doi.org/10.1021/acsami.9b16675
Karimzadeh Z, Mahmoudpour M, Rahimpour E, Jouyban A (2022) Nanomaterial based PVA nanocomposite hydrogels for biomedical sensing: advances toward designing the ideal flexible/wearable nanoprobes. Adv Colloid Interf Sci 305:102705. https://doi.org/10.1016/j.cis.2022.102705
Abudabbus MM, Jevremović I, Nešović K, Perić-Grujić A, Rhee KY, Mišković-Stanković V (2018) In situ electrochemical synthesis of silver-doped poly(vinyl alcohol)/graphene composite hydrogels and their physico-chemical and thermal properties. Compos Part B Eng 140:99–107. https://doi.org/10.1016/j.compositesb.2017.12.017
Devi LS, Palathinkal RP, Dasmahapatra AK (2024) Preparation of cross-linked PANI/PVA conductive hydrogels for electrochemical energy storage and sensing applications. Polymer (Guildf) 293:126673. https://doi.org/10.1016/j.polymer.2024.126673
Ahmadi M et al (2021) An investigation of affecting factors on MOF characteristics for biomedical applications: a systematic review. Heliyon 7(4):e06914. https://doi.org/10.1016/j.heliyon.2021.e06914
Song K et al (2022) Tailoring the crystal forms of the Ni-MOF catalysts for enhanced photocatalytic CO2-to-CO performance. Appl Catal B Environ 309:121232. https://doi.org/10.1016/j.apcatb.2022.121232
Pan Y et al (2022) Benzoic acid-modified 2D Ni-MOF for high-performance supercapacitors. Electrochim Acta 403:139679. https://doi.org/10.1016/j.electacta.2021.139679
Zhao H et al (2022) Electrocatalytic reduction of 4-nitrophenol over Ni-MOF/NF: understanding the self-enrichment effect of H-bonds. Chem Commun 58(31):4897–4900. https://doi.org/10.1039/D2CC00111J
Destruel P-L et al (2020) Novel in situ gelling ophthalmic drug delivery system based on gellan gum and hydroxyethylcellulose: innovative rheological characterization, in vitro and in vivo evidence of a sustained precorneal retention time. Int J Pharm 574:118734. https://doi.org/10.1016/j.ijpharm.2019.118734
**an H, Mengjun J, Yanyan Z, Ling H (2017) A silica/PVA adhesive hybrid material with high transparency, thermostability and mechanical strength. RSC Adv 7(5):2450–2459. https://doi.org/10.1039/C6RA25579E
Park H et al (2009) Effect of swelling ratio of injectable hydrogel composites on Chondrogenic differentiation of encapsulated rabbit marrow mesenchymal stem cells in vitro. Biomacromolecules 10(3):541–546. https://doi.org/10.1021/bm801197m
Liu Y, Wang Y, Chen Y, Wang C, Guo L (2020) NiCo-MOF nanosheets wrap** polypyrrole nanotubes for high-performance supercapacitors. Appl Surf Sci 507:145089. https://doi.org/10.1016/j.apsusc.2019.145089
Yang X et al (2023) NiCo-MOF Nanospheres created by the ultra-fast microwave method for use in high-performance Supercapacitors. Molecules 28(14):5613. https://doi.org/10.3390/molecules28145613
Singh N, Malik A, Nohwar S, Jana R, Mondal PC (2023) Covalent surface modification of nickel ferrite nanoparticles for electrochemical supercapacitor performance. New J Chem 47(11):5308–5315. https://doi.org/10.1039/D2NJ05566J
Sha R, Komori K, Badhulika S (2017) Graphene–polyaniline composite based ultra-sensitive electrochemical sensor for non-enzymatic detection of urea. Electrochim Acta 233:44–51. https://doi.org/10.1016/j.electacta.2017.03.043
Tran TQN, Das G, Yoon HH (2017) Nickel-metal organic framework/MWCNT composite electrode for non-enzymatic urea detection. Sensors Actuators B Chem 243:78–83. https://doi.org/10.1016/j.snb.2016.11.126
Zheng L et al (2019) Ni-P nanostructures on flexible paper for morphology effect of nonenzymatic electrocatalysis for urea. Electrochim Acta 320:134586. https://doi.org/10.1016/j.electacta.2019.134586
Yoon J, Lee E, Lee D, Oh T-S, Yoon YS, Kim D-J (2017) Communication—highly sensitive ag/ZnO Nanorods composite electrode for non-enzymatic urea detection. J Electrochem Soc 164(12):B558–B560. https://doi.org/10.1149/2.1341712JES/XML
Kumar THV, Sundramoorthy AK (2018) Non-enzymatic electrochemical detection of urea on silver nanoparticles anchored nitrogen-doped single-walled carbon nanotube modified electrode. J Electrochem Soc 165(8):B3006–B3016. https://doi.org/10.1149/2.0021808jes
Liu J, Siavash Moakhar R, Sudalaiyadum Perumal A, Roman HN, Mahshid S, Wachsmann-Hogiu S (2020) An AgNP-deposited commercial electrochemistry test strip as a platform for urea detection. Sci Rep 10(1):9527. https://doi.org/10.1038/s41598-020-66422-x
Amin S et al (2019) A practical non-enzymatic urea sensor based on NiCo 2 O 4 nanoneedles. RSC Adv 9(25):14443–14451. https://doi.org/10.1039/C9RA00909D
Bao C, Niu Q, Chen Z-A, Cao X, Wang H, Lu W (2019) Ultrathin nickel-metal–organic framework nanobelt based electrochemical sensor for the determination of urea in human body fluids. RSC Adv 9(50):29474–29481. https://doi.org/10.1039/C9RA05716A
Mangrio S et al (2023) Advanced Urea Precursors Driven NiCo2O4 Nanostructures Based Non-Enzymatic Urea Sensor for Milk and Urine Real Sample Applications. Biosens 13(4):444. https://doi.org/10.3390/BIOS13040444
Zhang H, Zhang Y, Wang M, Shen Y, Liu B (2020) Electro-oxidation of Urea on the Nickel Phosphate-based Nanomaterials. Int J Electrochem Sci 15(4):3400–3409. https://doi.org/10.20964/2020.04.02
Acknowledgments
S.B. acknowledges financial assistance from Department of Science and Technology (DST) Nano Mission project DST/ NM/ NT/2020/322.
Author information
Authors and Affiliations
Contributions
Gopika Mukundan – Conceptualization, Methodology, Data curation, Validation, Visualization, Writing- original draft preparation.
Sushmee Badhulika - Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary file 1
(DOCX 342 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mukundan, G., Badhulika, S. Nickel-cobalt metal-organic frameworks based flexible hydrogel as a wearable contact lens for electrochemical sensing of urea in tear samples. Microchim Acta 191, 252 (2024). https://doi.org/10.1007/s00604-024-06339-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06339-8