Abstract
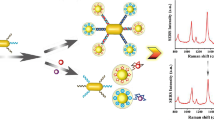
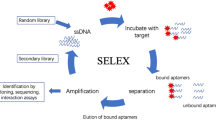
A new method is proposed for detecting typical melamine dopants in food using surface-enhanced Raman scattering (SERS) biosensing technology. Melamine specific aptamer was used as the identification probe, and gold magnets (AuNPs@MNPs) and small gold nanoparticles (AuNPs@MBA) were used as the basis for Raman detection. The Raman signal of the detection system can directly detect melamine quantitatively. Under optimized conditions, the detection of melamine was carried out in the low concentration range of 0.001–500 mg/kg, the enhancement factor (EF) was 2.3 × 107, and the detection limit was 0.001 mg/kg. The method is sensitive and rapid, and can be used for the rapid detection of melamine in the field environment.
Graphical Abstract






Similar content being viewed by others
Data availability
All data underlying this study are available in the article itself and its supplementary material.
References
**u CB, Klein KK (2010) Melamine in milk products in China: examining the factors that led to deliberate use of the contaminant. Food Policy 35(5):463–70. https://doi.org/10.1016/j.foodpol.2010.05.001
Tyan YC, Yang MH, Jong SB, Wang CK, Shiea J (2009) Melamine contamination. Anal Bioanal Chem 395(3):729–35. https://doi.org/10.1007/s00216-009-3009-0
Sharma K, Paradakar M (2010) The melamine adulteration scandal. Food Security. 2(1):97–107. https://doi.org/10.1007/s12571-009-0048-5
Faraji M, Kadijani PA (2016) Dabsyl chloride derivatisation of melamine followed by high-performance liquid chromatography determination in water samples. Int J Environ Anal Chem 96(15):1430–9. https://doi.org/10.1080/03067319.2016.1267160
Rovina K, Siddiquee S (2015) A review of recent advances in melamine detection techniques. J Food Compos Anal 43:25–38. https://doi.org/10.1016/j.jfca.2015.04.008
Chen MS, **g W, Lü SY, Li XJ, Wu HC, Lin BL et al (2011) Determination of melamine and related derivatives by high performance liquid chromatography. Chinese J Anal Chem 39(10):1572–6. https://doi.org/10.3724/sp.J.1096.2011.01572
Faraji M, Adeli M (2017) Sensitive determination of melamine in milk and powdered infant formula samples by high-performance liquid chromatography using Dabsyl chloride derivatization followed by dispersive liquid-liquid microextraction. Food Chem 221:139–46. https://doi.org/10.1016/j.foodchem.2016.10.002
Hon PYT, Chu PWS, Cheng CH, Lee TCL, Chan PK, Cheung STC et al (2011) Development of melamine certified reference material in milk using two different isotope dilution mass spectrometry techniques. J Chromatogr A. 1218(39):6907–13. https://doi.org/10.1016/j.chroma.2011.08.021
**a X, Ding SY, Li XW, Gong X, Zhang SX, Jiang HY et al (2009) Validation of a confirmatory method for the determination of melamine in egg by gas chromatography-mass spectrometry and ultra-performance liquid chromatography-tandem mass spectrometry. Anal Chimica Acta. 651(2):196–200. https://doi.org/10.1016/j.aca.2009.08.025
Fan YM, Ma XG, Li ZY, Chen MQ (2016) Fast derivatization followed by gas chromatography-mass spectrometry for simultaneous detection of melamine, ammeline, ammelide, and cyanuric acid in fish and shrimp. Food Anal Methods. 9(1):16–22. https://doi.org/10.1007/s12161-015-0171-1
Xu XM, Ren YP, Zhu Y, Cai ZX, Han JL, Huang BF et al (2009) Direct determination of melamine in dairy products by gas chromatography/mass spectrometry with coupled column separation. Anal Chimica Acta 650(1):39–43. https://doi.org/10.1016/j.aca.2009.04.026
Yang SP, Chen HW, Yang YL, Hu B, Zhang X, Zhou YF et al (2009) Imaging melamine in egg samples by surface desorption atmospheric pressure chemical ionization tandem mass spectrometry. Chinese J Anal Chem 37(3):315–8. https://doi.org/10.1016/s1872-2040(08)60088-2
Li M, Zhang LY, Meng ZH, Wang ZY, Wu H (2010) Molecularly-imprinted microspheres for selective extraction and determination of melamine in milk and feed using gas chromatography-mass spectrometry. J Chromatogr B-Anal Technol Biomed Life Sci 878(25):2333–8. https://doi.org/10.1016/j.jchromb.2010.07.003
Li J, Qi HY, Shi YP (2009) Determination of melamine residues in milk products by zirconia hollow fiber sorptive microextraction and gas chromatography-mass spectrometry. J Chromatogr A 1216(29):5467–71. https://doi.org/10.1016/j.chroma.2009.05.047
Gessei T, Arakawa T, Kudo H, Saito H, Mitsubayashi K (2014) Amperometric biosensor based on enzyme immobilization with post process for medical and multiple applications. Anal Letters 47(8):1361–74. https://doi.org/10.1080/00032719.2013.867496
Luong JHT, Male KB, Glennon JD (2008) Biosensor technology: technology push versus market pull. Biotechnol Adv 26(5):492–500. https://doi.org/10.1016/j.biotechadv.2008.05.007
Tan P, Li HS, Wang J, Gopinath SCB (2021) Silver nanoparticle in biosensor and bioimaging: clinical perspectives. Biotechnol Appl Biochem 68(6):1236–42. https://doi.org/10.1002/bab.2045
Dong YP, Luo XJ, Liu YQ, Yan CL, Li HX, Lv JC et al (2022) A disposable printed amperometric biosensor for clinical evaluation of creatinine in renal function detection. Talanta 248. https://doi.org/10.1016/j.talanta.2022.123592
Hidayat MA, Maharani DA, Purwanto DA, Kuswandi B, Yuwono M (2020) Simple and sensitive paper-based colorimetric biosensor for determining total polyphenol content of the green tea beverages. Biotechnol Bioprocess Eng 25(2):255–63. https://doi.org/10.1007/s12257-019-0299-8
Liu MH, Ma WX, Zhou YM, Liu B, Zhang XR, Zhang SS (2022) A label-free photoelectrochemical biosensor based on Crispr/ Cas12a system responsive deoxyribonucleic acid hydrogel and ?Click? Chem Acs Sensors. 7(10):3153–60. https://doi.org/10.1021/acssensors.2c01636
Tang YJ, Dai SY, Zhou YT, Cheng GF, He PG, Fang YZ (2019) A homogeneous electrochemical biosensor for detection of Mirna-21 based on DNA-templated click chemistry and catalytic hairpin assembly. Chinese J Anal Chem 47(7):1029–34. https://doi.org/10.19756/j.issn.0253-3820.191176
Yi ZH, Ren YS, Li Y, Li YN, Long F, Zhu AN (2023) Optical biosensors for microbial toxin detection: recent advances and future trends. Microchem J 191. https://doi.org/10.1016/j.microc.2023.108894
Gao GJ, Fan L, Lu HM, Hua YJ (2008) Engineering Deinococcus radiodurans into biosensor to monitor radioactivity and genotoxicity in environment. Chinese Sci Bullet 53(11):1675–81. https://doi.org/10.1007/s11434-008-0224-6
Hideshima S, Saito M, Fujita K, Harada Y, Tsuna M, Sekiguchi S et al (2018) Label-free detection of allergens in food via surfactant-induced signal amplification using a field effect transistor-based biosensor. Sensors Actuators B-Chem 254:1011–6. https://doi.org/10.1016/j.snb.2017.07.187
Pan MF, Yin ZJ, Liu KX, Du XL, Liu HL, Wang S (2019) Carbon-based nanomaterials in sensors for food safety. Nanomaterials 9(9). https://doi.org/10.3390/nano9091330
Wasito H, Fatoni A, Hermawan D, Susilowati SS (2019) Immobilized bacterial biosensor for rapid and effective monitoring of acute toxicity in water. Ecotoxicol Environ Saf 170:205–9. https://doi.org/10.1016/j.ecoenv.2018.11.141
Chen J, Luo ZW, Sun CJ, Huang ZJ, Zhou C, Yin S et al (2019) Research progress of DNA walker and its recent applications in biosensor. Trac-Trends in Anal Chem 120. https://doi.org/10.1016/j.trac.2019.115626
Mao ZF, Chen RP, Wang XJ, Zhou ZX, Peng Y, Li S et al (2022) Crispr/Cas12a-based technology: a powerful tool for biosensing in food safety. Trends Food Sci Technol 122:211–22. https://doi.org/10.1016/j.tifs.2022.02.030
Zhang RF, Wang Y, Qu XN, Li SS, Zhao YH, Liu S et al (2019) Exonuclease III-powered DNA walking machine for label-free and ultrasensitive electrochemical sensing of antibiotic. Sensors Actuators B-Chem 297. https://doi.org/10.1016/j.snb.2019.126771
Chen JJ, Sun X, Wang Y, Gao ZX, Zheng B (2023) Portable biosensor for cardiac troponin I based on the combination of a DNA walking machine and a personal glucose meter. Sensors Actuators B-Chem 385. https://doi.org/10.1016/j.snb.2023.133712
Mujica ML, Zhang YY, Bédioui F, Gutiérrez F, Rivas G (2020) Label-free graphene oxide-based Spr genosensor for the quantification of Microrna21. Anal Bioanal Chem 412(15):3539–46. https://doi.org/10.1007/s00216-020-02593-w
Sun X, Wang WY, Chai YY, Zheng Z, Wang Y, Bi J et al (2023) A DNA walker triggered isothermal amplification method based on freezing construction of AuNP probes and its application in ricin detection. Analyst 148(3):690–9. https://doi.org/10.1039/d2an01793h
Yun W, Zhu HH, Wu H, Zhuo L, Wang RQ, Ha X et al (2021) A "Turn-on" and proximity ligation assay dependent DNA Tweezer for one-step amplified fluorescent detection of DNA. Spectrochim Acta Part a-Mol Biomol Spectrosc 249. https://doi.org/10.1016/j.saa.2020.119292
Shang ZX, Ma PF, Khan IM, Zhang Y, Wang ZP (2023) A DNA tweezers fluorescence aptasensor based on split aptamer -assisted magnetic nanoparticles for the detection of enrofloxacin in food. Food Control 145. https://doi.org/10.1016/j.foodcont.2022.109437
Cui YB, Yan H, Sun Z, Ling Y, Luo HQ, Li NB (2023) A photoelectrochemical biosensor based on Znin2s4@AuNPs coupled with circular bipedal DNA walker for signal-on detection of circulating tumor DNA. Biosens Bioelectron 231. https://doi.org/10.1016/j.bios.2023.115295
**ong ZW, Ren YR, Wang C, Wu G, Yun W, Chen H et al (2022) A programmed, autonomous, and self-powered DNA motor for one-step amplification detection of ochratoxin A. Food Anal Methods. 15(4):847–55. https://doi.org/10.1007/s12161-021-02169-z
Qiao XJ, Ma X, Ma XY, Yue TL, Sheng QL (2021) A label-free aptasensor for ochratoxin A detection with signal amplification strategies on ultrathin micron-sized 2d Mof sheets. Sensors Actuators B-Chem 334. https://doi.org/10.1016/j.snb.2021.129682
Wang GA, Kong CP, Li F (2023) Design and bioanalytical applications of stochastic DNA walkers. Chem Commun 59(37):5492–501. https://doi.org/10.1039/d3cc00965c
Jiang J, Zhang P, Chai YQ, Yuan R, Peng KF (2021) Electrochemiluminescence biosensor based on a 3d DNA walking nanomachine with a powerful payload capacity. Sensors Actuators B-Chem 330. https://doi.org/10.1016/j.snb.2020.129337
Wang L, Liu ZJ, Cao HX, Liang GX (2021) Ultrasensitive colorimetric mirna detection based on magnetic 3d DNA walker and unmodified AuNPs. Sensors Actuators B-Chem 337. https://doi.org/10.1016/j.snb.2021.129813
Kelestemur S, Avci E, Çulha M (2018) Raman and surface-enhanced raman scattering for biofilm characterization. Chemosensors 6(1). https://doi.org/10.3390/chemosensors6010005
Luo ZX, Yang WS, Luo Y, Peng AD, Ma Y, Fu HB et al (2011) Potential-induced Raman behavior of individual R-Di-2-naphthylprolinol molecules on a Ag-modified Ag electrode. J Raman Spectrosc 42(5):951–7. https://doi.org/10.1002/jrs.2819
Furini LN, Sanchez-Cortes S, López-Tocón I, Otero JC, Aroca RF, Constantino CJL (2015) Detection and quantitative analysis of carbendazim herbicide on Ag nanoparticles via surface-enhanced Raman scattering. J Raman Spectrosc 46(11):1095–101. https://doi.org/10.1002/jrs.4737
Lee PC, Meisel D (1982) Adsorption and surface-enhanced raman of dyes on silver and gold sols. J Phys Chem (1952) 86(17):3391–5. https://doi.org/10.1021/j100214a025
Conde J, Baptista PV, Hernández Y, Sanz V, de la Fuente JM (2012) Modification of plasmid DNA topology by “histone-mimetic” gold nanoparticles. Nanomedicine. 7(11):1657–66. https://doi.org/10.2217/nnm.12.21
Zhou WL, Carpenter EE, Lin J, Kumbhar A, Sims J, O’Connor CJ (2001) Nanostructures of gold coated iron core-shell nanoparticles and the nanobands assembled under magnetic field. European Phys J D 16(1–3):289–92. https://doi.org/10.1007/s100530170112
Liu BW, Liu JW (2017) Freezing directed construction of bio/nano interfaces: reagentless conjugation, denser spherical nucleic acids, and better nanoflares. J Am Chem Soc 139(28):9471–4. https://doi.org/10.1021/jacs.7b04885
Wang ZG, Chen RP, Hou Y, Qin YK, Li S, Yang SP et al (2022) DNA hydrogels combined with microfluidic chips for melamine detection. Analytica Chimica Acta 1228. https://doi.org/10.1016/j.aca.2022.340312
Wei H, Xu HX (2013) Hot Spots in Different metal nanostructures for plasmon-enhanced Raman spectroscopy. Nanoscale. 5(22):10794–805. https://doi.org/10.1039/c3nr02924g
Wang XJ, Mao ZF, Chen RP, Li SN, Ren SY, Liang J et al (2022) Self-assembled DNA origami-based duplexed aptasensors combined with centrifugal filters for efficient and rechargeable Atp detection. Biosens Bioelectron 211. https://doi.org/10.1016/j.bios.2022.114336
Wang YH, Song W, Zhao HY, Ma X, Yang SY, Qiao XJ et al (2021) DNA walker-assisted aptasensor for highly sensitive determination of ochratoxin A. Biosens Bioelectron 182. https://doi.org/10.1016/j.bios.2021.113171
Yu J, Ai SH, Zhang WH, Wang C, Shi PF (2023) Ratiometric fluorescent aptasensor for convenient detection of ochratoxin A in beer and orange juice. Analyst. 148(20):5172–7. https://doi.org/10.1039/d3an01360j
Chen J, Huang YN, Yang XY, Zhang HJ, Li Z, Qin B et al (2018) Highly sensitive and visual detection of guanosine 3’-diphosphate-5’di(Tri)phosphate (Ppgpp) in bacteria based on copper ions-mediated 4-mercaptobenzoic acid modified gold nanoparticles. Anal Chimica Acta. 1023:89–95. https://doi.org/10.1016/j.aca.2018.02.082
Entzian C, Schubert T (2017) Map** the binding site of an aptamer on Atp using microscale thermophoresis. Jove-J Visualized Exp (119). https://doi.org/10.3791/55070
Jakab K, Melios N, Tsekenis G, Shaban A, Horváth V, Keresztes Z (2023) Comparative analysis of Ph and target-induced conformational changes of an oxytetracycline aptamer in solution phase and surface-immobilized form. Biomolecules 13(9). https://doi.org/10.3390/biom13091363
Jabbar AA, Wali LA, Alwan AM (2022) Improving the performance of chemical sensors using magnetic field. J Mater Sci-Mater Electron 33(32):24571–80. https://doi.org/10.1007/s10854-022-09168-8
Alwan AM, Wali LA, Hasan KK (2020) A new route for develo** highly efficient nano biochemical sensors for detecting ultra-low concentrations of tetracycline antibiotic residue in water. Gold Bullet 53(1):39–46. https://doi.org/10.1007/s13404-020-00272-3
Alwan AM, Wali LA, Yousif AA (2018) Optimization of AgNPs/Mesops active substrates for ultra-low molecule detection process. Silicon 10(5):2241–51. https://doi.org/10.1007/s12633-018-9758-7
Acknowledgments
I would like to express my heartfelt thanks to my tutor Zhixian Gao and teacher Shuyue Ren for their academic guidance, my Senior brother Ruipeng Chen for his writing help, and my classmates Jiaqi Lin, Han Cui and Rui Zhang for their summary.
Funding
The National Key Research and Development Program of China (2021YFA0910200) for funding this research project.
Author information
Authors and Affiliations
Contributions
Yu**g Ma: Conceptualization, Methodology, Validation, Writing—Original Draft, Writing—Review & Editing.
Han Cui: Resources, Formal analysis.
Ruipeng Chen: Formal analysis, Resources, Writing—Original Draft, Writing—Review & Editing.
Rui Zhang: Resources.
Jiaqi Lin: Resources, Formal analysis.
Shuyue Ren: Data Curation, Figure Processing, Investigation, Methodology, Formal analysis.
Jun Liang: Supervision, Project administration.
Zhixian Gao: Project administration, Funding acquisition.
Corresponding authors
Ethics declarations
This research did not involve human or animal samples.
Competing interests
The authors declare that no financial/personal interests or beliefs may affect their objectivity. Potential conflicts do not exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Y., Cui, H., Chen, R. et al. Rapid detection of melamine by DNA Walker mediated SERS sensing technique based on signal amplification function. Microchim Acta 191, 283 (2024). https://doi.org/10.1007/s00604-024-06336-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06336-x