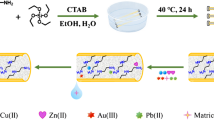
Abstract
The objective of this work was to develop an actinide-specific monolithic support in capillary designed to immobilize precise Pu:Am ratios and its coupling to inductively coupled plasma mass spectrometry (ICP-MS) for immobilized metal affinity chromatography applications. This format offers many advantages, such as reducing the sample amount and waste production, which are of prime importance when dealing with highly active radioelements. Four organic phosphorylated-based monoliths were synthesized in situ through UV photo-polymerization in capillary and characterized. The capillary coupling to ICP-MS was set up in conventional laboratory using Th and Sm as chemical analogues of Pu and Am. A dedicated method was developed to quantify online Th and Sm amounts immobilized on the monolithic capillaries, allowing to select the best monolith candidate poly(BMEP-co-EDMA)adp. By precisely adjusting the elemental composition in the loading solutions and applying the developed quantification method, the controlled immobilization of several Th:Sm molar ratios onto the monolith was successful. Finally, the capillary ICP-MS coupling was transposed in a glove box and by applying the strategy developed to design the monolithic support using Th and Sm, the immobilization of a 10.5 ± 0.2 (RSD = 2.3%, n = 3) Pu:Am molar ratio reflecting Pu ageing over 48 years was achieved in a controlled manner on poly(BMEP-co-EDMA)adp. Hence, the new affinity capillary monolithic support was validated, with only hundred nanograms or less of engaged radioelements and can be further exploited to precisely determine differential interactions of Pu and Am with targeted biomolecules in order to better anticipate the effect of Am on Pu biodistribution.
Graphical Abstract







Similar content being viewed by others
Data availability
The methodological approach is described step by step in the article, all elements are available for readers and users to be able to carry out similar studies.
References
ICRP (2019) Occupational intakes of radionuclides: part 4. ICRP publication 141. Ann IRCP 48(2/3):279–390
Van der Meeren A, Angulo JF, Bohand S, Griffiths NM (2019) A quick and simple in vitro assay to predict bioavailability of actinides following accidental exposure. Toxicol Vitro 58:142–149. https://doi.org/10.1016/j.tiv.2019.03.027
Van der Meeren A, Drouet G, Devilliers K, Laurent D, Moureau A, Feray A, Lamart S (2021) Evidence for a differential translocation of actinides across human lung epithelial cell monolayer in vitro according to their physicochemical properties and the presence of a chelating agent. Toxicol Vitro 70:105035. https://doi.org/10.1016/j.tiv.2020.105035
Van der Meeren A, Berthomieu C, Moureau A, Defrance M, Griffiths NM (2022) Use of an acellular assay to study interactions between actinides and biological or synthetic ligands. Biomolecules 12:1553. https://doi.org/10.3390/biom12111553
Porath J (1988) IMAC—immobilized metal ion affinity based chromatography. TrAC Trend Anal Chem 7:254–259. https://doi.org/10.1016/0165-9936(88)85074-X
Dedieu A, Bérenguer F, Basset C, Prat O, Quéméneur E, Pible O, Vidaud C (2009) Identification of uranyl binding proteins from human kidney-2 cell extracts by immobilized uranyl affinity chromatography and mass spectrometry. J Chromatogr A 1216:5365–5376. https://doi.org/10.1016/j.chroma.2009.05.023
Vidaud C, Robert M, Paredes E, Ortega R, Avazeri E, **g L, Guigonis JM, Bresson C, Malard V (2019) Deciphering the uranium target proteins in human dopaminergic SH-SY5Y cells. Arch Toxicol 93:2141–2154. https://doi.org/10.1007/s00204-019-02497-4
Vallet A et al (2023) The plasma membrane-associated cation-binding protein PCaP1 of Arabidopsis thaliana is a uranyl-binding protein. J Hazard Mater 446:130668. https://doi.org/10.1016/j.jhazmat.2022.130668
Aryal BP, Paunesku T, Woloschak GE, He C, Jensen MP (2012) A proteomic approach to identification of plutonium-binding proteins in mammalian cells. J Proteomics 75:1505–1514. https://doi.org/10.1016/j.jprot.2011.11.023
Svec F, Fréchet JMJ (1992) Continuous rods of macroporous polymer as high-performance liquid chromatography separation media. Anal Chem 64:820–822. https://doi.org/10.1021/ac00031a022
Nechvátalová M, Urban J (2022) Current trends in the development of polymer-based monolithic stationary phases. Anal Sci Adv 3:154–164. https://doi.org/10.1002/ansa.202100065
Gama MR, Rocha FRP, Bottoli CBG (2019) Monoliths: synthetic routes, functionalization and innovative analytical applications. TrAC Trends Anal Chem 115:39–51. https://doi.org/10.1016/j.trac.2019.03.020
Poddar S, Sharmeen S, Hage DS (2021) Affinity monolith chromatography: a review of general principles and recent developments. Electrophoresis 42:2577–2598. https://doi.org/10.1002/elps.202100163
Acquah C, Moy CKS, Danquah MK, Ongkudon CM (2016) Development and characteristics of polymer monoliths for advanced LC bioscreening applications: a review. J Chromatogr B 1015–1016:121–134. https://doi.org/10.1016/j.jchromb.2016.02.016
Pearson RG (1963) Hard and soft acids and bases. J Am Chem Soc 85:3533–3539. https://doi.org/10.1021/ja00905a001
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Crystallogr A 32:751. https://doi.org/10.1107/S0567739476001551
Choppin GR, Rizkalla EN (1994) Solution chemistry of actinides and lanthanides. In Gschneidner KA, Eyring JL, Lander GH, Choppin GR (eds) Handbook on the Physics and Chemistry of Rare Earths, Vol. 18 - Lanthanides/Actinides: Chemistry. Elsevier, Amsterdam, pp 559–590
Morgenstern A, Apostolidis C, Carlos-Marquez R, Mayer K, Molinet R (2002) Single-column extraction chromatographic separation of U, Pu, Np and Am. Radiochim Acta 90:81–85. https://doi.org/10.1524/ract.2002.90.2_2002.81
Chappa S, Das S, Debnath AK, Sahu M, Saxena MK, Pandey AK (2016) Spacer monomer in polymer chain influencing affinity of ethylene glycol methacrylate phosphate toward UO22+ and Pu4+ ions. Ind Eng Chem Res 55:8992–9002. https://doi.org/10.1021/acs.iecr.6b01534
Chappa S, Deb AKS, Ali SKM, Debnath AK, Aswal DK, Pandey AK (2016) Change in the affinity of ethylene glycol methacrylate phosphate monomer and its polymer anchored on a graphene oxide platform toward uranium(VI) and plutonium(IV) ions. J Phys Chem B 120:2942–2950. https://doi.org/10.1021/acs.jpcb.5b11293
Bresson C, Garcia-Cortes M, Vidaud C, Tran T (2022) Method for preparing a monolithic support on which uranyl cations are immobilised, and associated methods for capture and recovery. US Patent 2022/0339628A1
Di T, Tan D, Yu Q, Lin J, Zhu T, Li T, Li L (2019) Ultra-High Performance of hyper-crosslinked phosphate-based polymer for uranium and rare earth element adsorption in aqueous solution. Langmuir 35:13860–13871. https://doi.org/10.1021/acs.langmuir.9b02459
Paul S, Pandey AK, Shah RV, Bhushan KS, Aggarwal SK (2016) Polymer based sorbent materials for thermal ionization mass spectrometric determination of uranium(vi) and plutonium(iv) ions. J Anal Atom Spectrom 31:985–993. https://doi.org/10.1039/C5JA00463B
Mhatre AM, Chappa S, Chavan V, Pandey AK (2017) Thin film of poly(bis[2-(methacryloyloxy)ethyl]phosphate) grafted on surface of poly(ether sulfone) membrane for plutonium(IV)-selective alpha tracks registration in CR-39 detector. J Radioanal Nucl Ch 314:187–196
Mhatre AM, Chappa S, Paul S, Pandey AK (2017) Phosphate-bearing polymer grafted glass for plutonium(IV) ion-selective alpha spectrometry. J Anal Atom Spectrom 32:1566–1570. https://doi.org/10.1039/C7JA00156H
Krishna MVB, Chappa S, Chandrasekaran K, Karunasagar D, Pandey AK (2018) Determination of thorium and uranium in nickel-based alloys by ICP-MS after matrix separation using novel co-polymer of ethylene glycol methacrylate and bis[2-methacryloyloxy)ethyl] phosphate. At Spectrosc 39:142–150
Dong J, Zhou H, Wu R, Ye M, Zou H (2007) Specific capture of phosphopeptides by Zr4+ modified monolithic capillary column. J Sep Sci 30:2917–2923. https://doi.org/10.1002/jssc.200700350
Dziomba S, Araya-Farias M, Taverna M, Guerrouache M, Carbonnier B, Tran NT (2017) Microscope-assisted UV-initiated preparation of well-defined porous polymer monolithic plugs in glass microchips for peptide preconcentration. Anal Bioanal Chem 409:2155–2162. https://doi.org/10.1007/s00216-016-0161-1
Shao H, Reider B, Jarvas G, Guttman A, Jiang Z, Tran NT, Taverna M (2020) On-line enrichment of N-glycans by immobilized metal-affinity monolith for capillary electrophoresis analysis. Anal Chim Acta 1134:1–9. https://doi.org/10.1016/j.aca.2020.08.002
Chen ML, Li LM, Yuan BF, Ma Q, Feng YQ (2012) Preparation and characterization of methacrylate-based monolith for capillary hydrophilic interaction chromatography. J Chromatogr A 1230:54–60. https://doi.org/10.1016/j.chroma.2012.01.065
Chen X, Tolley HD, Lee ML (2011) Monolithic capillary columns synthesized from a single phosphate-containing dimethacrylate monomer for cation-exchange chromatography of peptides and proteins. J Chromatogr A 1218:4322–4331. https://doi.org/10.1016/j.chroma.2011.04.074
Chen X, Tolley HD, Lee ML (2010) Polymeric cation-exchange monolithic columns containing phosphoric acid functional groups for capillary liquid chromatography of peptides and proteins. J Chromatogr A 1217:3844–3854. https://doi.org/10.1016/j.chroma.2010.04.032
Wang F, Dong J, Jiang X, Ye M, Zou H (2007) Capillary trap column with strong cation-exchange monolith for automated shotgun proteome analysis. Anal Chem 79:6599–6606. https://doi.org/10.1021/ac070736f
Wang H, Duan J, Xu H, Zhao L, Liang Y, Shan Y, Zhang L, Liang Z, Zhang Y (2011) Monoliths with immobilized zirconium ions for selective enrichment of phosphopeptides. J Sep Sci 34:2113–2121. https://doi.org/10.1002/jssc.201100168
Viklund C, Svec F, Irgum K (1996) Monolithic, ‘molded’, porous materials with high flow characteristics for separations, catalysis, or solid-phase chemistry: control of porous properties during polymerization. Chem Mater 8:744–750. https://doi.org/10.1021/cm950437j
Choppin GR, Jensen MP (2010) Actinides in solution: complexation and kinetics. In: Morss LR, Edelstein NM, Fuger J (eds) The Chemistry of the Actinide and Transactinide Elements, 4th edn. Springer, Dordrecht, pp 2524–2621
Acknowledgements
The authors would like to acknowledge the FOCUSDEM R&D program on decommissioning and dismantling of CEA, the French Atomic Energy and Alternative Energies Commission, for financial support. The authors would like to also thank Marina Amaral Saraiva for her support for the setup of the capillary/ICP-MS coupling assembly in the glove box.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barhoum, S., Garcia-Cortes, M., Boudias, M. et al. Immobilization of controlled Pu:Am ratio on actinide-specific affinity monolith support developed in capillary and coupled to inductively coupled plasma mass spectrometry. Microchim Acta 191, 191 (2024). https://doi.org/10.1007/s00604-024-06274-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06274-8