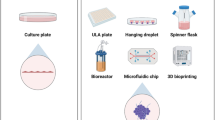
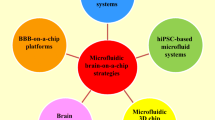
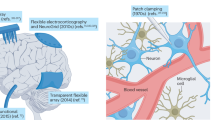
Abstract
The complex structure and function of the human central nervous system that develops from the neural tube made in vitro modeling quite challenging until the discovery of brain organoids. Human-induced pluripotent stem cells–derived brain organoids offer recapitulation of the features of early human neurodevelopment in vitro, including the generation, proliferation, and differentiation into mature neurons and micro-macroglial cells, as well as the complex interactions among these diverse cell types of the develo** brain. Recent advancements in brain organoids, microfluidic systems, real-time sensing technologies, and their cutting-edge integrated use provide excellent models and tools for emulation of fundamental neurodevelopmental processes, the pathology of neurological disorders, personalized transplantation therapy, and high-throughput neurotoxicity testing by bridging the gap between two-dimensional models and the complex three-dimensional environment in vivo. In this review, we summarize how bioengineering approaches are applied to mitigate the limitations of brain organoids for biomedical and clinical research. We further provide an extensive overview and future perspectives of the humanized brain organoids-on-chip platforms with integrated sensors toward brain organoid intelligence and biocomputing studies. Such approaches might pave the way for increasing approvable clinical applications by solving their current limitations.
Graphical Abstract




Similar content being viewed by others
References
Saglam-Metiner P, Gulce-Iz S, Biray-Avci C (2019) Bioengineering-inspired three-dimensional culture systems: organoids to create tumor microenvironment. Gene 686:203–212. https://doi.org/10.1016/j.gene.2018.11.058
Tang XY, Wu S, Wang D et al (2022) Human organoids in basic research and clinical applications. Signal Transduct Target Ther 7. https://doi.org/10.1038/s41392-022-01024-9
Lancaster MA, Renner M, Martin CA et al (2013) Cerebral organoids model human brain development and microcephaly. Nature 501:373–379. https://doi.org/10.1038/nature12517
Tran HN, Gautam V (2022) Micro/nano devices for integration with human brain organoids. Biosens Bioelectron 218:114750. https://doi.org/10.1016/j.bios.2022.114750
Jeong E, Choi S, Cho SW (2022) Recent advances in brain organoid technology for human brain research. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.2c17467
Pașca SP, Arlotta P, Bateup HS et al (2022) A nomenclature consensus for nervous system organoids and assembloids. Nature 609:907–910. https://doi.org/10.1038/s41586-022-05219-6
Tan SY, Feng X, Cheng LKW, Wu AR (2023) Vascularized human brain organoid on-chip. Lab Chip 23:2693–2709. https://doi.org/10.1039/d2lc01109c
Saglam-metiner P, Devamoglu U, Filiz Y, et al (2023) Spatio-temporal dynamics enhance cellular diversity, neuronal function and further maturation of human cerebral organoids. Commun Biol 6. https://doi.org/10.1038/s42003-023-04547-1
Cho AN, ** Y, An Y et al (2021) Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat Commun 12. https://doi.org/10.1038/s41467-021-24775-5
Smirnova L, Caffo BS, Gracias DH et al (2023) Organoid intelligence (OI): the new frontier in biocomputing and intelligence-in-a-dish. Front Sci 1:1–23. https://doi.org/10.3389/fsci.2023.1017235
Morales Pantoja IE, Smirnova L, Muotri AR et al (2023) First organoid intelligence (OI) workshop to form an OI community. Front Artif Intell 6. https://doi.org/10.3389/frai.2023.1116870
Cai H, Ao Z, Tian C et al (2023) Brain organoid computing for artificial intelligence. bioRxiv Prepr Serv Biol. https://doi.org/10.1101/2023.02.28.530502
Bossink EGBM, Zakharova M, De Bruijn DS et al (2021) Measuring barrier function in organ-on-chips with cleanroom-free integration of multiplexable electrodes. Lab Chip 21:2040–2049. https://doi.org/10.1039/d0lc01289k
Kang SY, Kimura M, Shrestha S et al (2023) A pillar and perfusion plate platform for robust human organoid culture and analysis. Adv Healthc Mater 2302502:1–16. https://doi.org/10.1002/adhm.202302502
Zheng Y, Zhang F, Xu S, Wu L (2021) Advances in neural organoid systems and their application in neurotoxicity testing of environmental chemicals. Genes Environ 43:1–12. https://doi.org/10.1186/s41021-021-00214-1
Spitz S, Bolognin S, Brandauer K et al (2022) Development of a multi-sensor integrated midbrain organoid-on- a-chip platform for studying Parkinson’s disease. https://doi.org/10.1101/2022.08.19.504522
Song J, Bang S, Choi N, Kim HN (2022) Brain organoid-on-a-chip: a next-generation human brain avatar for recapitulating human brain physiology and pathology. Biomicrofluidics 16. https://doi.org/10.1063/5.0121476
Castiglione H, Vigneron PA, Baquerre C et al (2022) Human brain organoids-on-chip: advances, challenges, and perspectives for preclinical applications. Pharmaceutics 14. https://doi.org/10.3390/pharmaceutics14112301
Park J, Lee BK, Jeong GS et al (2015) Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of Alzheimer’s disease. Lab Chip 15:141–150. https://doi.org/10.1039/c4lc00962b
Zhu Y, Wang L, Yu H et al (2017) In situ generation of human brain organoids on a micropillar array. Lab Chip 17:2941–2950. https://doi.org/10.1039/C7LC00682A
Zhu Y, Wang L, Yin F et al (2017) Probing impaired neurogenesis in human brain organoids exposed to alcohol. Integr Biol 9:968–978. https://doi.org/10.1039/c7ib00105c
Yin F, Zhu Y, Wang Y, Qin J (2018) Engineering brain organoids to probe impaired neurogenesis induced by cadmium. ACS Biomater Sci Eng 4:1908–1915. https://doi.org/10.1021/acsbiomaterials.8b00160
Berger E, Magliaro C, Paczia N et al (2018) Millifluidic culture improves human midbrain organoid vitality and differentiation. Lab Chip 18:3172–3183. https://doi.org/10.1039/c8lc00206a
Karzbrun E, Kshirsagar A, Cohen SR et al (2018) Human brain organoids on a chip reveal the physics of folding. Nat Phys 14:515–522. https://doi.org/10.1038/s41567-018-0046-7
Wang Y, Wang L, Guo Y et al (2018) Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. RSC Adv 8:1677–1685. https://doi.org/10.1039/c7ra11714k
Wang Y, Wang L, Zhu Y, Qin J (2018) Human brain organoid-on-a-chip to model prenatal nicotine exposure. Lab Chip 18:851–860. https://doi.org/10.1039/c7lc01084b
Ao Z, Cai H, Havert DJ et al (2020) One-stop microfluidic assembly of human brain organoids to model prenatal cannabis exposure. Anal Chem 92:4630–4638. https://doi.org/10.1021/acs.analchem.0c00205
Rifes P, Isaksson M, Rathore GS et al (2020) Modeling neural tube development by differentiation of human embryonic stem cells in a microfluidic WNT gradient. Nat Biotechnol 38:1265–1273. https://doi.org/10.1038/s41587-020-0525-0
Cui K, Wang Y, Zhu Y et al (2020) Neurodevelopmental impairment induced by prenatal valproic acid exposure shown with the human cortical organoid-on-a-chip model. Microsystems Nanoeng 6.https://doi.org/10.1038/s41378-020-0165-z
Ao Z, Cai H, Wu Z et al (2021) Tubular human brain organoids to model microglia-mediated neuroinflammation. Lab Chip 21:2751–2762. https://doi.org/10.1039/d1lc00030f
Ao Z, Song S, Tian C et al (2022) Understanding immune-driven brain aging by human brain organoid microphysiological analysis platform. Adv Sci 9:1–10. https://doi.org/10.1002/advs.202200475
Cui K, Chen W, Cao R et al (2022) Brain organoid-on-chip system to study the effects of breast cancer derived exosomes on the neurodevelopment of brain. Cell Regen 11:1–12. https://doi.org/10.1186/s13619-021-00102-7
Salmon I, Grebenyuk S, Abdel Fattah AR et al (2022) Engineering neurovascular organoids with 3D printed microfluidic chips. Lab Chip 22:1615–1629. https://doi.org/10.1039/d1lc00535a
Seiler ST, Mantalas GL, Selberg J et al (2022) Modular automated microfluidic cell culture platform reduces glycolytic stress in cerebral cortex organoids. Sci Rep 12:1–12. https://doi.org/10.1038/s41598-022-20096-9
Zhu Y, Zhang X, Sun L et al (2023) Engineering human brain assembloids by microfluidics. Adv Mater 2210083:1–8. https://doi.org/10.1002/adma.202210083
Passaro AP, Stice SL (2021) Electrophysiological analysis of brain organoids: current approaches and advancements. Front Neurosci 14:1–13. https://doi.org/10.3389/fnins.2020.622137
Chung WG, Kim E, Song H et al (2022) Recent advances in electrophysiological recording platforms for brain and heart organoids. Adv NanoBiomed Res 2.https://doi.org/10.1002/anbr.202200081
Zhou Y, Song H, Ming G li (2023) Genetics of human brain development. Nat Rev Genet. https://doi.org/10.1038/s41576-023-00626-5
Taverna E, Götz M, Huttner WB (2014) The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu Rev Cell Dev Biol 30:465–502. https://doi.org/10.1146/annurev-cellbio-101011-155801
Stoufflet J, Tielens S, Nguyen L (2023) Sha** the cerebral cortex by cellular crosstalk. Cell 186:2733–2747. https://doi.org/10.1016/j.cell.2023.05.040
Kim J, Koo BK, Knoblich JA (2020) Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol 21:571–584. https://doi.org/10.1038/s41580-020-0259-3
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. 2:663–676. https://doi.org/10.1016/j.cell.2006.07.024
Soldner F, Jaenisch R (2019) Stem cells, genome editing, and the path to translational medicine. 175:615–632. https://doi.org/10.1016/j.cell.2018.09.010.Stem
Kelley KW, Pașca SP (2022) Human brain organogenesis: toward a cellular understanding of development and disease. Cell 185:42–61. https://doi.org/10.1016/j.cell.2021.10.003
Lokai T, Albin B, Qubbaj K et al (2023) A review on current brain organoid technologies from a biomedical engineering perspective. Exp Neurol 367:114461. https://doi.org/10.1016/j.expneurol.2023.114461
Eichmüller OL, Knoblich JA (2022) Human cerebral organoids — a new tool for clinical neurology research. Nat Rev Neurol 18:661–680. https://doi.org/10.1038/s41582-022-00723-9
Levy RJ, Paşca SP (2022) What have organoids and assembloids taught us about the pathophysiology of neuropsychiatric disorders? Biol Psychiatry 93:632–641. https://doi.org/10.1016/j.biopsych.2022.11.017
Steeg R, Mueller SC, Mah N et al (2021) EBiSC best practice: how to ensure optimal generation, qualification, and distribution of iPSC lines. Stem Cell Reports 16:1853–1867. https://doi.org/10.1016/j.stemcr.2021.07.009
Gonzalez C, Armijo E, Bravo-Alegria J et al (2017) Modeling amyloid beta and tau pathology in human cerebral organoids. Physiol Behav 176:139–148. https://doi.org/10.1038/s41380-018-0229-8.Modeling
Arber C, Toombs J, Lovejoy C et al (2020) Familial Alzheimer’s disease patient-derived neurons reveal distinct mutation-specific effects on amyloid beta. Mol Psychiatry 25:2919–2931. https://doi.org/10.1038/s41380-019-0410-8
Quadrato G, Nguyen T, Macosko EZ et al (2017) Cell diversity and network dynamics in photosensitive human brain. Nature 42:2920–2921. https://doi.org/10.1038/nature22047
Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, Paulsen B, Nguyen L, Adiconis X, Regev A, Levin JZ, Arlotta P (2019) Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570(7762):523–527. https://doi.org/10.1038/s41586-019-1289-x
Sidhaye J, Knoblich JA (2021) Brain organoids: an ensemble of bioassays to investigate human neurodevelopment and disease. Cell Death Differ 28:52–67. https://doi.org/10.1038/s41418-020-0566-4
Sun N, Meng X, Liu Y et al (2021) Applications of brain organoids in neurodevelopment and neurological diseases. J Biomed Sci 28:1–16. https://doi.org/10.1186/s12929-021-00728-4
Adlakha YK (2023) Human 3D brain organoids: steering the demolecularization of brain and neurological diseases. Cell Death Discov 9. https://doi.org/10.1038/s41420-023-01523-w
Cederquist GY, Tchieu J, Callahan SJ et al (2020) A multiplex human pluripotent stem cell platform defines molecular and functional subclasses of autism-related genes. Cell Stem Cell 27:35-49.e6. https://doi.org/10.1016/j.stem.2020.06.004
Hofmann B, Zinöcker S, Holm S et al (2022) Organoids in the clinic: a systematic review of outcomes. Cells Tissues Organs 499–511. https://doi.org/10.1159/000527237
Zhao Z, Chen X, Dowbaj AM et al (2022) Organoids. Nat Rev Methods Prim 2. https://doi.org/10.1038/s43586-022-00174-y
Hernández D, Rooney LA, Daniszewski M et al (2022) Culture variabilities of human iPSC-derived cerebral organoids are a major issue for the modelling of phenotypes observed in Alzheimer’s disease. Stem Cell Rev Reports 18:718–731. https://doi.org/10.1007/s12015-021-10147-5
Jensen KB, Little MH (2023) Organoids are not organs: sources of variation and misinformation in organoid biology. Stem Cell Reports 18:1255–1270. https://doi.org/10.1016/j.stemcr.2023.05.009
Chiaradia I, Lancaster MA (2020) Brain organoids for the study of human neurobiology at the interface of in vitro and in vivo. Nat Neurosci 23:1496–1508. https://doi.org/10.1038/s41593-020-00730-3
Saglam-Metiner P, Duran E, Sabour-Takanlou L et al (2023) Differentiation of neurons, astrocytes, oligodendrocytes and microglia from human induced pluripotent stem cells to form neural tissue-on-chip: a neuroinflammation model to evaluate the therapeutic potential of extracellular vesicles derived from mesenchymal stem cells. Stem Cell Rev Reports. https://doi.org/10.1007/s12015-023-10645-8
Ormel PR, Vieira de Sá R, van Bodegraven EJ et al (2018) Microglia innately develop within cerebral organoids. Nat Commun 9. https://doi.org/10.1038/s41467-018-06684-2
Amin ND, Kelley KW, Amin ND et al (2023) Generating human neural diversity with a multiplexed morphogen screen in organoids. bioRxiv 2023.05.31.541819
Qian X, Song H, Ming GL (2019) Brain organoids: advances, applications and challenges. Dev 146. https://doi.org/10.1242/dev.166074
Reumann D, Krauditsch C, Novatchkova M et al (2023) In vitro modeling of the human dopaminergic system using spatially arranged ventral midbrain-striatum-cortex assembloids. Nat Methods 20:2–5. https://doi.org/10.1038/s41592-023-02080-x
Legnini I, Emmenegger L, Zappulo A et al (2023) Spatiotemporal, optogenetic control of gene expression in organoids. Nat Methods 20:1544–1552. https://doi.org/10.1038/s41592-023-01986-w
Fiorenzano A, Sozzi E, Birtele M et al (2021) Single-cell transcriptomics captures features of human midbrain development and dopamine neuron diversity in brain organoids. Nat Commun 12:1–19. https://doi.org/10.1038/s41467-021-27464-5
Silva CG, Peyre E, Nguyen L (2019) Cell migration promotes dynamic cellular interactions to control cerebral cortex morphogenesis. Nat Rev Neurosci 20:318–329. https://doi.org/10.1038/s41583-019-0148-y
Garritsen O, van Battum EY, Grossouw LM, Pasterkamp RJ (2023) Development, wiring and function of dopamine neuron subtypes. Nat Rev Neurosci 24:134–152. https://doi.org/10.1038/s41583-022-00669-3
Brignani S, Raj DDA, Schmidt ERE et al (2020) Remotely produced and axon-derived netrin-1 instructs GABAergic neuron migration and dopaminergic substantia nigra development. Neuron 107:684-702.e9. https://doi.org/10.1016/j.neuron.2020.05.037
Smits LM, Magni S, Kinugawa K et al (2020) Single-cell transcriptomics reveals multiple neuronal cell types in human midbrain-specific organoids. Cell Tissue Res 382:463–476. https://doi.org/10.1007/s00441-020-03249-y
Walter J, Bolognin S, Poovathingal SK et al (2021) The Parkinson’s-disease-associated mutation LRRK2-G2019S alters dopaminergic differentiation dynamics via NR2F1. Cell Rep 37. https://doi.org/10.1016/j.celrep.2021.109864
Zagare A, Barmpa K, Smajic S et al (2022) Midbrain organoids mimic early embryonic neurodevelopment and recapitulate LRRK2-p.Gly2019Ser-associated gene expression. Am J Hum Genet 109:311–327. https://doi.org/10.1016/j.ajhg.2021.12.009
Bagley JA, Reumann D, Bian S et al (2017) Fused cerebral organoids model interactions between brain regions. Nat Methods 14:743–751. https://doi.org/10.1038/nmeth.4304
Sabate-Soler S, Nickels SL, Saraiva C et al (2022) Microglia integration into human midbrain organoids leads to increased neuronal maturation and functionality. Glia 70:1267–1288. https://doi.org/10.1002/glia.24167
Birey F, Andersen J, Makinson CD et al (2017) Assembly of functional forebrain spheroids from human pluripotent cells. Nature 545:54–59. https://doi.org/10.1038/nature22330.Assembly
**ang Y, Tanaka Y, Patterson B, et al (2018) Fusion of regionally-specified hPSC-derived organoids models human. 21:383–398. https://doi.org/10.1016/j.stem.2017.07.007.Fusion
**ang Y, Tanaka Y, Cakir B et al (2020) hESC-derived thalamic organoids form reciprocal projections when fused with cortical organoids. 24:487–497.https://doi.org/10.1016/j.stem.2018.12.015.hESC-derived
Miura Y, Li MY, Birey F et al (2020) Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat Biotechnol 38:1421–1430. https://doi.org/10.1038/s41587-020-00763-w
Miura Y, Li MY, Revah O et al (2022) Engineering brain assembloids to interrogate human neural circuits. Nat Protoc 17:15–35. https://doi.org/10.1038/s41596-021-00632-z
Martins F, Miguel J, Fischer C et al (2020) Self-organizing 3D human trunk neuromuscular organoids. Cell Stem Cell 26:172-186.e6. https://doi.org/10.1016/j.stem.2019.12.007
Ao Z, Cai H, Wu Z et al (2021) Controllable fusion of human brain organoids using acoustofluidics. Lab Chip 4. https://doi.org/10.1039/D0LC01141J
Park DS, Kozaki T, Tiwari SK et al (2023) iPS-cell-derived microglia promote brain organoid maturation via cholesterol transfer. Nature 623:397–405. https://doi.org/10.1038/s41586-023-06713-1
Abud EM, Ramirez RN, Martinez ES et al (2017) iPSC-derived human microglia-like cells to study neurological diseases. Neuron 94:278-293.e9. https://doi.org/10.1016/j.neuron.2017.03.042
Pham MT, Pollock KM, Rose MD et al (2018) Generation of human vascularized brain organoids. NeuroReport 29:588–593. https://doi.org/10.1097/WNR.0000000000001014
Shi Y, Sun L, Wang M et al (2020) Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol 18:1–29. https://doi.org/10.1371/journal.pbio.3000705
Sun XY, Ju XC, Li Y et al (2022) Generation of vascularized brain organoids to study neurovascular interactions. Elife 11:1–28. https://doi.org/10.7554/eLife.76707
McComish SF, MacMahon Copas AN, Caldwell MA (2022) Human brain-based models provide a powerful tool for the advancement of Parkinson’s disease research and therapeutic development. Front Neurosci 16:1–15. https://doi.org/10.3389/fnins.2022.851058
Jusop AS, Thanaskody K, Tye GJ et al (2023) Development of brain organoid technology derived from iPSC for the neurodegenerative disease modelling: a glance through. Front Mol Neurosci 16:1–14. https://doi.org/10.3389/fnmol.2023.1173433
Wang SN, Wang Z, Xu TY et al (2020) Cerebral organoids repair ischemic stroke brain injury. Transl Stroke Res 11:983–1000. https://doi.org/10.1007/s12975-019-00773-0
Qian X, Nguyen HN, Song MM et al (2016) Brain region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165:1238–1254. https://doi.org/10.1016/j.cell.2016.04.032. Brain
Pellegrini L, Bonfio C, Chadwick J et al (2020) Human CNS barrier-forming organoids with cerebrospinal fluid production. Science (80- ) 369. https://doi.org/10.1126/science.aaz5626
Matsui T, Shinozawa T (2021) Human organoids for predictive toxicology research and drug development. Front Genet 12:1–14. https://doi.org/10.3389/fgene.2021.767621
Fan P, Wang YH, Xu M et al (2022) The application of brain organoids in assessing neural toxicity. Front Mol Neurosci 15:1–11. https://doi.org/10.3389/fnmol.2022.799397
Lee JA, Bae DH, Choi WH et al (2022) Effects of sevoflurane exposure on fetal brain development using cerebral organoids. J Mol Neurosci 72:2440–2450. https://doi.org/10.1007/s12031-022-02080-0
Schwartz MP, Hou Z, Propson NE et al (2015) Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc Natl Acad Sci U S A 112:12516–12521. https://doi.org/10.1073/pnas.1516645112
Hong Y, Dong X, Chang L et al (2023) Microglia-containing cerebral organoids derived from induced pluripotent stem cells for the study of neurological diseases. iScience 26:106267. https://doi.org/10.1016/j.isci.2023.106267
Yaldiz B, Saglam-Metiner P, Yesil-Celiktas O (2022) Decellularised extracellular matrix-based biomaterials for repair and regeneration of central nervous system. Expert Rev Mol Med 23:1–11. https://doi.org/10.1017/erm.2021.22
Srinivasan B, Kolli AR, Esch MB et al (2015) TEER measurement techniques for in vitro barrier model systems. J Lab Autom 20:107–126
Skardal A, Murphy S V, Devarasetty M et al (2017) Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep 7. https://doi.org/10.1038/s41598-017-08879-x
Marrero D, Guimera A, Maes L et al (2023) Organ-on-a-chip with integrated semitransparent organic electrodes for barrier function monitoring. Lab Chip 23:1825–1834. https://doi.org/10.1039/d2lc01097f
Shin SR, Zhang YS, Kim DJ et al (2016) Aptamer-based microfluidic electrochemical biosensor for monitoring cell-secreted trace cardiac biomarkers. Anal Chem 88:10019–10027. https://doi.org/10.1021/acs.analchem.6b02028
Dornhof J, Kieninger J, Muralidharan H et al (2022) Microfluidic organ-on-chip system for multi-analyte monitoring of metabolites in 3D cell cultures. Lab Chip 22:225–239. https://doi.org/10.1039/d1lc00689d
Shaegh SAM, Ferrari F De, Zhang YS, et al (2016) A microfluidic optical platform for real-time monitoring of pH and oxygen in microfluidic bioreactors and organ-on-chip devices. Biomicrofluidics 10. https://doi.org/10.1063/1.4955155
Zoio P, Lopes-Ventura S, Oliva A (2022) Biomimetic full-thickness skin-on-a-chip based on a fibroblast-derived matrix. Micro 2:191–211. https://doi.org/10.3390/micro2010013
Zoio P, Lopes-Ventura S, Oliva A (2021) Barrier-on-a-chip with a modular architecture and integrated sensors for real-time measurement of biological barrier function. Micromachines 12. https://doi.org/10.3390/mi12070816
Azizgolshani H, Coppeta JR, Vedula EM et al (2021) High-throughput organ-on-chip platform with integrated programmable fluid flow and real-time sensing for complex tissue models in drug development workflows. Lab Chip 21:1454–1474. https://doi.org/10.1039/d1lc00067e
Henry OYF, Villenave R, Cronce MJ et al (2017) Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function. Lab Chip 17:2264–2271. https://doi.org/10.1039/c7lc00155j
Zhang X, Wang W, Nordin AN et al (2017) The influence of the electrode dimension on the detection sensitivity of electric cell–substrate impedance sensing (ECIS) and its mathematical modeling. Sensors Actuators, B Chem 247:780–790. https://doi.org/10.1016/j.snb.2017.03.047
Partel S, Dincer C, Kasemann S et al (2016) Lift-off free fabrication approach for periodic structures with tunable nano gaps for interdigitated electrode arrays. ACS Nano 10:1086–1092. https://doi.org/10.1021/acsnano.5b06405
Badiola-Mateos M, Di Giuseppe D, Paoli R et al (2021) A novel multi-frequency trans-endothelial electrical resistance (MTEER) sensor array to monitor blood-brain barrier integrity. Sensors Actuators, B Chem 334:129599. https://doi.org/10.1016/j.snb.2021.129599
Aydogmus H, Hu M, Ivancevic L et al (2023) An organ-on-chip device with integrated charge sensors and recording microelectrodes. Sci Rep 13:8062. https://doi.org/10.1038/s41598-023-34786-5
Cui F, Zhou Z, Zhou HS (2020) Review—measurement and analysis of cancer biomarkers based on electrochemical biosensors. J Electrochem Soc 167:37525. https://doi.org/10.1149/2.0252003jes
Mariani F, Gualandi I, Schuhmann W, Scavetta E (2022) Micro- and nano-devices for electrochemical sensing. Springer Vienna
Modena MM, Chawla K, Misun PM, Hierlemann A (2018) Smart cell culture systems: integration of sensors and actuators into microphysiological systems. ACS Chem Biol 13:1767–1784
Shin SR, Kilic T, Zhang YS et al (2017) Label-free and regenerative electrochemical microfluidic biosensors for continual monitoring of cell secretomes. Adv Sci 4. https://doi.org/10.1002/advs.201600522
Asif A, Park SH, Soomro AM et al (2021) Microphysiological system with continuous analysis of albumin for hepatotoxicity modeling and drug screening. J Ind Eng Chem 98:318–326. https://doi.org/10.1016/j.jiec.2021.03.035
Bavli D, Prill S, Ezra E et al (2016) Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of Mitochondrial dysfunction. Proc Natl Acad Sci U S A 113:E2231–E2240. https://doi.org/10.1073/pnas.1522556113
Tanumihardja E, Rodríguez AP, Loessberg-Zahl JT et al (2021) On-chip electrocatalytic NO sensing using ruthenium oxide nanorods. Sensors Actuators, B Chem 334. https://doi.org/10.1016/j.snb.2021.129631
Li LM, Wang XY, Hu LS et al (2012) Vascular lumen simulation and highly-sensitive nitric oxide detection using three-dimensional gelatin chip coupled to TiC/C nanowire arrays microelectrode. Lab Chip 12:4249–4256. https://doi.org/10.1039/c2lc40148g
Rivera KR, Yokus MA, Erb PD et al (2019) Measuring and regulating oxygen levels in microphysiological systems: design, material, and sensor considerations. Analyst 144:3190–3215
Clark LC, Wolf R, Granger D, Taylor Z (1953) Continuous recording of blood oxygen tensions by polarography. 6:189–193. https://doi.org/10.1152/JAPPL.1953.6.3.189
Liebisch F, Weltin A, Marzioch J et al (2020) Zero-consumption Clark-type microsensor for oxygen monitoring in cell culture and organ-on-chip systems. Sensors Actuators B Chem 322:128652. https://doi.org/10.1016/J.SNB.2020.128652
Zhang YS, Aleman J, Shin SR et al (2017) Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc Natl Acad Sci U S A 114:E2293–E2302. https://doi.org/10.1073/pnas.1612906114
Aleman J, Kilic T, Mille LS et al (2021) Microfluidic integration of regeneratable electrochemical affinity-based biosensors for continual monitoring of organ-on-a-chip devices. Nat Protoc 16:2564–2593
Kann SH, Shaughnessey EM, Coppeta JR et al (2022) Measurement of oxygen consumption rates of human renal proximal tubule cells in an array of organ-on-chip devices to monitor drug-induced metabolic shifts. Microsystems Nanoeng 8. https://doi.org/10.1038/s41378-022-00442-7
Kann SH, Shaughnessey EM, Zhang X et al (2023) Steady-state monitoring of oxygen in a high-throughput organ-on-chip platform enables rapid and non-invasive assessment of drug-induced nephrotoxicity. Analyst. https://doi.org/10.1039/d3an00380a
Ortega MA, Rodríguez-Comas J, Yavas O et al (2021) In situ LSPR sensing of secreted insulin in organ-on-chip. Biosensors 11:1–14. https://doi.org/10.3390/bios11050138
Chen C, Wang J (2020) Optical biosensors: an exhaustive and comprehensive review. Anal R Soc Chem 5:1605–1628. https://doi.org/10.1039/C9AN01998G
Fuchs S, Johansson S, Tjell A et al (2021) In-line analysis of organ-on-chip systems with sensors: integration, fabrication, challenges, and potential. ACS Biomater Sci Eng 7:2926–2948. https://doi.org/10.1021/acsbiomaterials.0c01110
Mou L, Mandal K, Mecwan MM et al (2022) Integrated biosensors for monitoring microphysiological systems. Lab Chip 22:3801–3816
Chmayssem A, Verplanck N, Tanase CE et al (2021) Development of a multiparametric (bio)sensing platform for continuous monitoring of stress metabolites. Talanta 229. https://doi.org/10.1016/j.talanta.2021.122275
Ortega MA, Fernández-Garibay X, Castaño AG et al (2019) Muscle-on-a-chip with an on-site multiplexed biosensing system for: in situ monitoring of secreted IL-6 and TNF-α. Lab Chip 19:2568–2580. https://doi.org/10.1039/c9lc00285e
Park TE, Mustafaoglu N, Herland A et al (2019) Hypoxia-enhanced blood-brain barrier chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat Commun 10:1–12. https://doi.org/10.1038/s41467-019-10588-0
Kadry H, Noorani B, Cucullo L (2020) A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 17:1–24. https://doi.org/10.1186/s12987-020-00230-3
Galea I (2021) The blood–brain barrier in systemic infection and inflammation. Cell Mol Immunol 18:2489–2501. https://doi.org/10.1038/s41423-021-00757-x
Cecen B, Saygili E, Zare I et al (2023) Biosensor integrated brain-on-a-chip platforms: progress and prospects in clinical translation. Biosens Bioelectron 225. https://doi.org/10.1016/j.bios.2023.115100
Cakir B, **ang Y, Tanaka Y et al (2019) Development of human brain organoids with functional vascular like system. Nat Methods 16:1169–1175. https://doi.org/10.1038/s41592-019-0586-5.Development
Wang YI, Hasan Erbil Abacia MLS (2017) Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening ying. Physiol Behav 176:139–148. https://doi.org/10.1002/bit.26045.Microfluidic
Huang Q, Tang B, Romero JC et al (2022) Shell microelectrode arrays (MEAs) for brain organoids. Sci Adv 8. https://doi.org/10.1126/sciadv.abq5031
Soscia DA, Lam D, Tooker AC et al (2020) A flexible 3-dimensional microelectrode array for: in vitro brain models. Lab Chip 20:901–911. https://doi.org/10.1039/c9lc01148j
Park Y, Franz CK, Ryu H et al (2021) Three-dimensional, multifunctional neural interfaces for cortical spheroids and engineered assembloids. Sci Adv 7. https://doi.org/10.1126/sciadv.abf9153
Landry CR, Yip MC, Zhou Y et al (2023) Electrophysiological and morphological characterization of single neurons in intact human brain organoids. J Neurosci Methods 394:1–22. https://doi.org/10.1016/j.jneumeth.2023.109898
Kalmykov A, Huang C, Bliley J et al (2019) Organ-on-e-chip: three-dimensional self-rolled biosensor array for electrical interrogations of human electrogenic spheroids. Sci Adv 5:729–752
Nasr B, Chatterton R, Yong JHM et al (2018) Self-organized nanostructure modified microelectrode for sensitive electrochemical glutamate detection in stem cells-derived brain organoids. Biosensors 8. https://doi.org/10.3390/bios8010014
Miny L, Maisonneuve BGC, Quadrio I, Honegger T (2022) Modeling neurodegenerative diseases using in vitro compartmentalized microfluidic devices. Front Bioeng Biotechnol 10:1–17. https://doi.org/10.3389/fbioe.2022.919646
Bardy C, van den Hurk M, Kakaradov B et al (2016) Predicting the functional states of human iPSC-derived neurons with single-cell RNA-seq and electrophysiology Cedric. Physiol Behav 176:139–148. https://doi.org/10.1038/mp.2016.158.Predicting
Moutaux E, Charlot B, Genoux A et al (2018) An integrated microfluidic/microelectrode array for the study of activity-dependent intracellular dynamics in neuronal networks. Lab Chip 12. https://doi.org/10.1039/C8LC00694F
Kalmykov A, Reddy JW, Bedoyan E et al (2021) Bioelectrical interfaces with cortical spheroids in three-dimensions. J Neural Eng 18. https://doi.org/10.1088/1741-2552/abf290
Pediaditakis I, Kodella KR, Manatakis DV et al (2021) Modeling alpha-synuclein pathology in a human brain-chip to assess blood-brain barrier disruption. Nat Commun 12:1–17. https://doi.org/10.1038/s41467-021-26066-5
Lee M-H, Liu K-T, Thomas JL et al (2020) Peptide-imprinted poly(hydroxymethyl 3,4-ethylenedioxythiophene) nanotubes for detection of α synuclein in human brain organoids. ACS Appl Nano Mater. https://doi.org/10.1021/acsanm.0c01476
Li C, Fleck JS, Martins-Costa C et al (2023) Single-cell brain organoid screening identifies developmental defects in autism. Nature 621:373–380. https://doi.org/10.1038/s41586-023-06473-y
Paulsen B, Velasco S, Kedaigle AJ et al (2022) Autism genes converge on asynchronous development of shared neuron classes. Springer, US
Yang Y, Yang R, Kang B et al (2023) Single-cell long-read sequencing in human cerebral organoids uncovers cell-type-specific and autism-associated exons. Cell Rep 42:113335. https://doi.org/10.1016/j.celrep.2023.113335
Kirischuk S (2022) Kee** excitation – inhibition ratio in balance. Int J Mol Sci Rev 23:5746. https://doi.org/10.3390/ijms23105746
Sohal VS, Rubenstein JLR (2019) Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol Psychiatry 24:1248–1257. https://doi.org/10.1038/s41380-019-0426-0
Li Y, Tang P, Cai S et al (2020) Organoid based personalized medicine: from bench to bedside. Cell Regeneration
Mayhew CN, Singhania R (2023) A review of protocols for brain organoids and applications for disease modeling. STAR Protoc 4:101860. https://doi.org/10.1016/j.xpro.2022.101860
Yaldiz B, Saglam-Metiner P, Cam SB et al (2021) Effect of sterilization methods on the mechanical stability and extracellular matrix constituents of decellularized brain tissues. J Supercrit Fluids 175:105299. https://doi.org/10.1016/j.supflu.2021.105299
Kabay G, Manz A, Dincer C (2022) Microfluidic roadmap for translational nanotheranostics. Small Methods 6. https://doi.org/10.1002/smtd.202101217
Young AT, Rivera KR, Erb PD, Daniele MA (2019) Monitoring of microphysiological systems: integrating sensors and real-time data analysis toward autonomous decision-making. ACS Sensors 4:1454–1464
Saorin G, Caligiuri I, Rizzolio F (2023) Microfluidic organoids-on-a-chip: the future of human models. Semin Cell Dev Biol 144:41–54. https://doi.org/10.1016/j.semcdb.2022.10.001
Rajan SAP, Aleman J, Wan MM et al (2020) Probing prodrug metabolism and reciprocal toxicity with an integrated and humanized multi-tissue organ-on-a-chip platform. Acta Biomater 106:124–135. https://doi.org/10.1016/j.actbio.2020.02.015
Funding
This work is supported by the Scientific and Technological Research Council (TUBITAK) of Turkey through 119M578. This study is partially conducted under the OrChESTRA (Organ-on-a-Chip Focused Strategic Partnership) project, which has received funding from the European Union’s Horizon Europe’s research and innovation program under Grant Agreement No. 101079473. P.S-M. gratefully acknowledges the TUBITAK 2211-A National Graduate Scholarship Program and 2214-A International Doctoral Research Fellowship Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saglam-Metiner, P., Yildirim, E., Dincer, C. et al. Humanized brain organoids-on-chip integrated with sensors for screening neuronal activity and neurotoxicity. Microchim Acta 191, 71 (2024). https://doi.org/10.1007/s00604-023-06165-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06165-4