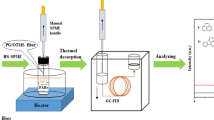
Abstract
In order to improve the extraction ability of carbon fibers (CFs) for microextraction of polycyclic aromatic hydrocarbons (PAHs), biochar nanospheres derived from glucose were in-situ grown onto the surface of CFs via hydrothermal synthesis. The surface morphology and elemental composition of biochar nanospheres-CFs were investigated by scanning electron microscopy and X-ray photoelectron spectroscopy. Thereafter, the biochar nanosphere-CFs were pulled into the polyetheretherketone tube for solid-phase microextraction, and the tube was combined with high-performance liquid chromatography-diode array detector to online detect PAHs. With the help of π-stacking, hydrophobic, and hydrophilic effect of biochar nanospheres, the extraction efficiency of CFs was greatly enhanced (enrichment factor increased by 293% compared with the original). The conditions affecting the analytical performance (sampling volume, sampling rate, methanol content, and desorption time) were investigated. Under the optimal conditions, an online analytical method for microextraction and determination of several PAHs was developed, and satisfactory results were achieved. The limits of detection were 0.003-0.010 ng mL-1 owing to high enrichment effect (2973-3600), linearity ranged from 0.010-15.0 ng mL-1, and relative standard deviations were 0.4%-1.6% (intra-day) and 2.4%-4.4% (inter-day), respectively. The method was applied to analyze environmental water samples (rain water, snow water, and river water), and spiked recoveries within 80.0%-119% were obtained.
Graphical abstract







Similar content being viewed by others
Reference
Leng G, Chen W-J, Xu W-B, Wang Y (2017) Fully automated vortex-assisted liquid-liquid microextraction coupled to gas chromatography-mass spectrometry for the determination of trace levels of phthalate esters in liquor samples. Food Anal Method 10(9):3071–3078. https://doi.org/10.1007/s12161-017-0874-6
Xu R, Gao HT, Zhu F, Cao WX, Yan YH, Zhou X, Xu Q, Ji WL (2016) SPE-UPLC-MS/MS for the determination of phthalate monoesters in rats urine and its application to study the effects of food emulsifier on the bioavailability of priority controlling PAEs. J Chromatogr B 1012–1013:97–105. https://doi.org/10.1016/j.jchromb.2016.01.007
Arthur CL, Pawliszyn J (1990) Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62(19):2145–2148. https://doi.org/10.1021/ac00218a019
Abolghasemi MM, Parastari S, Yousefi V (2014) Microextraction of phenolic compounds using a fiber coated with a polyaniline-montmorillonite nanocomposite. Microchim Acta 182(1–2):273–280. https://doi.org/10.1007/s00604-014-1323-5
Kataoka H (2021) In-tube solid-phase microextraction: Current trends and future perspectives. J Chromatogr A 1636:461787. https://doi.org/10.1016/j.chroma.2020.461787
Zhang J, Zhang W, Bao T, Chen Z (2015) Polydopamine-based immobilization of zeolitic imidazolate framework-8 for in-tube solid-phase microextraction. J Chromatogr A 1388:9–16. https://doi.org/10.1016/j.chroma.2015.02.010
Zhao S, Wang H-T, Li K, Zhang J, Wang X-Y, Guo G-S (2018) Fast determination of residual sulfonamides in milk by in-tube solid-phase microextraction coupled with capillary electrophoresis-laser induced fluorescence. Chinese J Anal Chem 46(3):e1810–e1816. https://doi.org/10.1016/s1872-2040(17)61076-4
Ahmadi SH, Manbohi A, Heydar KT (2015) Electrochemically controlled in-tube solid phase microextraction of naproxen from urine samples using an experimental design. Analyst 140(2):497–505. https://doi.org/10.1039/c4an01664e
Naing NN, Yau Li SF, Lee HK (2016) Magnetic micro-solid-phase-extraction of polycyclic aromatic hydrocarbons in water. J Chromatogr A 1440:23–30. https://doi.org/10.1016/j.chroma.2016.02.046
Zhang Y, Wang N, Lu Z, Chen N, Cui C, Chen X (2022) Smart titanium wire used for the evaluation of hydrophobic/hydrophilic interaction by in-tube solid phase microextraction. Molecules 27:2353. https://doi.org/10.3390/molecules27072353
Mahmoudpour M, Mohtadinia J, Mousavi M-M, Ansarin M, Nemati M (2017) Application of the microwave-assisted extraction and dispersive liquid–liquid microextraction for the analysis of PAHs in smoked rice. Food Anal Method 10:277–286. https://doi.org/10.1007/s12161-016-0579-2
Ji X, Sun M, Li C, Han S, Guo W, Feng J (2019) Carbonized silk fibers for in-tube solid-phase microextraction to detect polycyclic aromatic hydrocarbons in water samples. J Sep Sci 42:3535–3543. https://doi.org/10.1002/jssc.201900426
Feng J, Sun M, Bu Y, Luo C (2016) Development of a cheap and accessible carbon fibers-in-poly(ether ether ketone) tube with high stability for online in-tube solid-phase microextraction. Talanta 148:313–320. https://doi.org/10.1016/j.talanta.2015.11.001
Wang X, Feng J, Tian Y, Li C, Ji X, Luo C, Sun M (2019) Melamine-formaldehyde aerogel functionalized with polydopamine as in-tube solid-phase microextraction coating for the determination of phthalate esters. Talanta 199:317–323. https://doi.org/10.1016/j.talanta.2019.02.081
Feng J, Wang X, Tian Y, Bu Y, Luo C, Sun M (2017) Electrophoretic deposition of graphene oxide onto carbon fibers for in-tube solid-phase microextraction. J Chromatogr A 1517:209–214. https://doi.org/10.1016/j.chroma.2017.07.086
Jon C, Zou Y, Zhao J, Ri H, Wang L, Kaw H, Meng L, Shang H, Li D (2020) Simultaneous determination of multiple phytohormones in tomato by ionic liquid-functionalized carbon fibers-based solid-phase microextraction coupled with liquid chromatography-mass spectrometry. Anal Chim Acta 1137:143–155. https://doi.org/10.1016/j.aca.2020.09.050
Ghaemi F, Amiri A, Yunus R (2014) Methods for coating solid-phase microextraction fibers with carbon nanotubes. Trac-Trend Anal Chem. 59:133–143. https://doi.org/10.1016/j.trac.2014.04.011
Sakai-Otsuka Y, Ogawa Y, Satoh T, Chen WC, Borsali R (2021) Carbohydrate-attached fullerene derivative for selective localization in ordered carbohydrate-block-poly(3-hexylthiophene) nanodomains. Carbohyd Polym 255:117528. https://doi.org/10.1016/j.carbpol.2020.117528
Wang Y, Ngoc Pham T, Tian Y, Morikawa Y, Yan L (2021) Density functional theory study on a nitrogen-rich carbon nitride material C(3)N(5) as photocatalyst for CO(2) reduction to C1 and C2 products. J Colloid Interf Sci 585:740–749. https://doi.org/10.1016/j.jcis.2020.10.054
Rovani S, Medeiros LF, Lima EC, Fernandes AN (2019) Application of biochar from agro-industrial waste in solid-phase extraction for the determination of 17β-estradiol from aqueous solution. Int J Environ Sci Te 16(12):7623–7630. https://doi.org/10.1007/s13762-019-02295-6
Lyu H, Zhang Q, Shen B (2020) Application of biochar and its composites in catalysis. Chemosphere 240:124842. https://doi.org/10.1016/j.chemosphere.2019.124842
Yin L, Hu QK, Mondal S, Xu JQ, Ouyang GF (2019) Peanut shell-derived biochar materials for effective solid-phase microextraction of polycyclic aromatic hydrocarbons in environmental waters. Talanta 202:90–95. https://doi.org/10.1016/j.talanta.2019.04.020
Chen J, Zhang Z, Yu J, Tang S, Cui B, Zeng J (2022) Solid phase microextraction of benzenes in river water by pomelo peel biochar. Chin J Chromatogr 40(11):1031–1038. https://doi.org/10.3724/SP.J.1123.2022.02006
Li J, Chang R, Wang F, Zhao G (2016) A facile solid-phase micro-extraction fiber based on pine needles biochar coating for extraction of polychlorinated biphenyls from water samples. Chromatographia 79(15–16):1033–1040. https://doi.org/10.1007/s10337-016-3118-9
He J, Ma Z, Yang Y, Hemar Y, Zhao T (2020) Extraction of tetracycline in food samples using biochar microspheres prepared by a Pickering emulsion method. Food Chem 329:127162. https://doi.org/10.1016/j.foodchem.2020.127162
Sun M, Feng J, Feng J, Sun H, Feng Y, Ji X, Li C, Han S, Sun M (2022) Biochar nanosphere- and covalent organic framework nanosphere-functionalized titanium dioxide nanorod arrays on carbon fibers for solid-phase microextraction of organic pollutants. Chem Eng J 433:133645. https://doi.org/10.1016/j.cej.2021.133645
Sun M, Feng J, Ji X, Li C, Han S, Sun M, Feng Y, Feng J, Sun H (2021) Polyaniline/titanium dioxide nanorods functionalized carbon fibers for in-tube solid-phase microextraction of phthalate esters prior to high performance liquid chromatography-diode array detection. J Chromatogr A 1642:462003. https://doi.org/10.1016/j.chroma.2021.462003
Zhang S, Li Z, Yang X, Wang C, Wang Z (2015) Fabrication of a three-dimensional graphene coating for solid-phase microextraction of polycyclic aromatic hydrocarbons. RSC Adv 5(67):54329–54337. https://doi.org/10.1039/c5ra05616k
Wang X, Liu L, Wang X, Xu G, Zhao R, Wang M, Lin J, Wang X (2021) High crystalline magnetic covalent organic framework with three-dimensional grapevine structure for ultrasensitive extraction of nitro-polycyclic aromatic hydrocarbons in food and environmental samples. Food Chem 361:130018. https://doi.org/10.1016/j.foodchem.2021.130018
Li J, Ma L, Tang M, Xu L (2013) C12-Ag wire as solid-phase microextraction fiber for determination of benzophenone ultraviolet filters in river water. J Chromatogr A 1298:1–8. https://doi.org/10.1016/j.chroma.2013.05.010
Zakerian R, Bahar S (2019) Electrochemical exfoliation of pencil graphite for preparation of graphene coating as a new versatile SPME fiber for determination of polycyclic aromatic hydrocarbons by gas chromatography. Microchim Acta 186(12):861. https://doi.org/10.1007/s00604-019-3851-5
Wang F, Zheng Y, Qiu J, Liu S, Tong Y, Zhu F, Ouyang G (2018) Graphene-based metal and nitrogen-doped carbon composites as adsorbents for highly sensitive solid phase microextraction of polycyclic aromatic hydrocarbons. Nanoscale 10(21):10073–10078. https://doi.org/10.1039/c8nr01910j
Mauri-Aucejo A, Amoros P, Moragues A, Guillem C, Belenguer-Sapina C (2016) Comparison of the solid-phase extraction efficiency of a bounded and an included cyclodextrin-silica microporous composite for polycyclic aromatic hydrocarbons determination in water samples. Talanta 156–157:95–103. https://doi.org/10.1016/j.talanta.2016.05.011
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC, Nos. 21777054 and 21405061) and the Shandong Provincial Natural Science Foundation of China (No. ZR2019MB058).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, M., Ji, X., Sun, M. et al. Biochar nanosphere-functionalized carbon fibers for in-tube solid-phase microextraction of polycyclic aromatic hydrocarbons in environmental water followed by liquid chromatography and diode array detection. Microchim Acta 190, 395 (2023). https://doi.org/10.1007/s00604-023-05982-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05982-x