Abstract
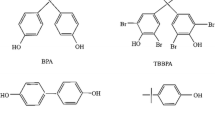
A core–satellite-structured surface molecularly imprinted polymer has been synthesized for the enrichment of 3-phenoxybenzaldehyde by pipette tip solid-phase extraction (SPE). In a typical sol–gel process, two silane reagents as functional monomers and 3-phenoxybenzoic acid as the dummy template, the surface imprinting layer was coated on the core–satellite silica microsphere, which formed the core–satellite-structured molecularly imprinted polymer (CSMIP). Compared to the silica-based core–shell ones, this CS-MIP exhibits a stunning surface area (142 m2 g−1) in micrometer size and also overcomes the aggregation trends of core–shell structure in nanoscale. Taking potassium permanganate solution as oxidizer and indicator, the adsorbed 3-phenoxybenzaldehyde can be a quantitatively determined through redox reaction after elution. The value of maximum adsorption capacity and imprinting factor of CS-MIP were calculated to be 87.5 μg mg−1 and 2.13, respectively. These CS-MIPs were packed into commercial pipette tip as the sorbent to concentrate 3-phenoxybenzaldehyde. Under the optimum condition, a liner relationship was achieved in the range 0.200 to 1.00 μg mL−1 and the limit of detection was 81 ng mL−1. Moreover, this customized SPE device exhibits good adsorption capability after six sequential adsorption–desorption cycles, and the high recovery range of 92.2~99.7% of spiked tap water assay demonstrated its potential application for real sample analysis.

Schematic presentation of core–satellite molecularly imprinted polymer preparation strategy and customized pipette tip solid-phase extraction device.





Similar content being viewed by others
References
Tang W, Wang D, Wang J, Wu Z, Li L, Huang M, Xu S, Yan D (2018) Pyrethroid pesticide residues in the global environment: an overview. Chemosphere 191:990–1007. https://doi.org/10.1016/j.chemosphere.2017.10.115
Wheelock CE, Miller JL, Miller MJ, Gee SJ, Shan G, Hammock BD (2004) Development of toxicity identification evaluation procedures for pyrethroid detection using esterase activity. Environ Toxicol Chem 23(11):2699–2708. https://doi.org/10.1897/03-544
Zhan H, Huang Y, Lin Z, Bhatt P, Chen S (2020) New insights into the microbial degradation and catalytic mechanism of synthetic pyrethroids. Environ Res 182:109138. https://doi.org/10.1016/j.envres.2020.109138
Wang X, Martínez M-A, Dai M, Chen D, Ares I, Romero A, Castellano V, Martínez M, Rodríguez JL, Martínez-Larrañaga M-R, Anadón A, Yuan Z (2016) Permethrin-induced oxidative stress and toxicity and metabolism. A review. Environ Res 149:86–104. https://doi.org/10.1016/j.envres.2016.05.003
Shan G, Huang H, Stoutamire DW, Gee SJ, Leng G, Hammock BD (2004) A sensitive class specific immunoassay for the detection of pyrethroid metabolites in human urine. Chem Res Toxicol 17(2):218–225. https://doi.org/10.1021/tx034220c
Ahn KC, Lohstroh P, Gee SJ, Gee NA, Lasley B, Hammock BD (2007) High-throughput automated luminescent magnetic particle-based immunoassay to monitor human exposure to pyrethroid insecticides. Anal Chem 79(23):8883–8890. https://doi.org/10.1021/ac070675l
Richards J, Lu Z, Fu Q, Schlenk D, Gan J (2017) Conversion of pyrethroid insecticides to 3-phenoxybenzoic acid on urban hard surfaces. Environ Sci Technol Lett 4(12):546–550. https://doi.org/10.1021/acs.estlett.7b00466
Vadhana D, Carloni M, Fedeli D, Nasuti C, Gabbianelli R (2011) Perturbation of rat heart plasma membrane fluidity due to metabolites of permethrin insecticide. Cardiovasc Toxicol 11(3):226–234. https://doi.org/10.1007/s12012-011-9116-0
Rosita G, Manuel C, Franco M, Cinzia N, Donatella F, Emiliano L, Luca M, Roberta G (2015) Permethrin and its metabolites affect Cu/Zn superoxide conformation: fluorescence and in silico evidences. Mol BioSyst 11(1):208–217. https://doi.org/10.1039/C4MB00491D
Cycoń M, Żmijowska A, Piotrowska-Seget Z (2014) Enhancement of deltamethrin degradation by soil bioaugmentation with two different strains of Serratia marcescens. Int J Environ Sci Technol 11(5):1305–1316. https://doi.org/10.1007/s13762-013-0322-0
Birolli WG, Alvarenga N, Seleghim MHR, Porto ALM (2016) Biodegradation of the pyrethroid pesticide esfenvalerate by marine-derived fungi. Mar Biotechnol 18(4):511–520. https://doi.org/10.1007/s10126-016-9710-z
Xu C, Li X, ** M, Sun X, Niu L, Lin C, Liu W (2018) Early life exposure of zebrafish (Danio rerio) to synthetic pyrethroids and their metabolites: a comparison of phenotypic and behavioral indicators and gene expression involved in the HPT axis and innate immune system. Environ Sci Pollut Res 25(13):12992–13003. https://doi.org/10.1007/s11356-018-1542-0
Kumar KS, Swaroop BL, Suvardhan K, Rekha D, Jayaraj B, Chiranjeevi P (2006) Facile and sensitive spectrophotometric determination of synthetic pyrithroids in their formulations, water and grain samples. Environ Monit Assess 122(1):1–8. https://doi.org/10.1007/s10661-005-9159-4
Gouda AA, Al Mazroai LSJMGC (2014) Sensitive spectrophotometric determination of cypermethrin in its formulations, water and environmental samples. Main Group Chem 13(3):233–242
Saeidi M, Yazdani Z, Sabermahani F (2015) Simultaneous derivatization/pre-concentration of 3-phenoxybenzaldehyde as transformation product of permethrin with 2,4-dinitrophenylhydrazine by solid phase extraction and spectrophotometric detection. J Anal Chem 70(1):17–21. https://doi.org/10.1134/S1061934815010104
Zhang Y, Lian X, Yan B (2020) A dual-functional intelligent logic detector based on new Ln-MOFs: first visual logical probe for the two-dimensional monitoring of pyrethroid biomarkers. J Mater Chem 8(9):3023–3028. https://doi.org/10.1039/c9tc06335h
Pandey V, Chauhan A, Pandey G, Mudiam MKR (2015) Optical sensing of 3-phenoxybenzoic acid as a pyrethroid pesticides exposure marker by surface imprinting polymer capped on manganese-doped zinc sulfide quantum dots. Anal Chem Res 5:21–27 https://doi.org/10.1016/j.ancr.2015.06.002
Farooq S, Nie J, Cheng Y, Yan Z, Li J, Bacha SAS, Mushtaq A, Zhang H (2018) Molecularly imprinted polymers’ application in pesticide residue detection. Analyst 143(17):3971–3989. https://doi.org/10.1039/C8AN00907D
Ye T, Yin W, Zhu N, Yuan M, Cao H, Yu J, Gou Z, Wang X, Zhu H, Reyihanguli A, Xu F (2018) Colorimetric detection of pyrethroid metabolite by using surface molecularly imprinted polymer. Sensors Actuators B Chem 254:417–423. https://doi.org/10.1016/j.snb.2017.07.132
Ge J, Zhang Q, Zhang T, Yin Y (2008) Core–satellite nanocomposite catalysts protected by a porous silica shell: controllable reactivity, high stability, and magnetic recyclability. Angew Chem Int Ed 47(46):8924–8928. https://doi.org/10.1002/anie.200803968
Bagwe RP, Hilliard LR, Tan W (2006) Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir 22(9):4357–4362. https://doi.org/10.1021/la052797j
Moczko E, Guerreiro A, Piletska E, Piletsky S (2013) PEG-stabilized core-shell surface-imprinted nanoparticles. Langmuir 29(31):9891–9896. https://doi.org/10.1021/la401891f
Adham A, Harald R, Peter M, Haifei Z (2012) One-pot synthesis of spheres-on-sphere silica particles from a single precursor for fast HPLC with low back pressure. Adv Mater 24(45):6042–6048. https://doi.org/10.1002/adma.201202810
Wu J, Tu W, Zhang Y, Guo B, Li S, Zhang Y, Wang Y, Pan M (2017) Poly-dopamine coated graphite oxide/silicon composite as anode of lithium ion batteries. Powder Technol 311:200–205. https://doi.org/10.1016/j.powtec.2017.01.063
Qu Y, Qin L, Liu X, Yang Y (2020) Reasonable design and sifting of microporous carbon nanosphere-based surface molecularly imprinted polymer for selective removal of phenol from wastewater. Chemosphere 251:126376. https://doi.org/10.1016/j.chemosphere.2020.126376
Gauthier M, Mazouzi D, Reyter D, Lestriez B, Moreau P, Guyomard D, Roué L (2013) A low-cost and high performance ball-milled Si-based negative electrode for high-energy Li-ion batteries. Energy Environ Sci 6(7):2145–2155. https://doi.org/10.1039/C3EE41318G
Liu J, Pan J, Ma Y, Liu S, Qiu F, Yan Y (2018) A versatile strategy to fabricate dual-imprinted porous adsorbent for efficient treatment co-contamination of λ-cyhalothrin and copper(II). Chem Eng J 332:517–527. https://doi.org/10.1016/j.cej.2017.09.079
He P, Zhu H, Ma Y, Liu N, Niu X, Wei M, Pan J (2019) Rational design and fabrication of surface molecularly imprinted polymers based on multi-boronic acid sites for selective capture glycoproteins. Chem Eng J 367:55–63. https://doi.org/10.1016/j.cej.2019.02.140
Wang P, Liu J, Chen X, Ma X, Guo D, Li Z, Pan J (2019) Janus silica nanosheets-based MMIPs platform for synergetic selective capture and fast separation of 2′-deoxyadenosine: two different components segmented on the surface of one object. Chem Eng J 369:793–802. https://doi.org/10.1016/j.cej.2019.03.175
Hua S, Zhao L, Cao L, Wang X, Gao J, Xu C (2018) Fabrication and evaluation of hollow surface molecularly imprinted polymer for rapid and selective adsorption of dibenzothiophene. Chem Eng J 345:414–424. https://doi.org/10.1016/j.cej.2018.03.128
Yao J, Ma Y, Liu J, Liu S, Pan J (2019) Janus-like boronate affinity magnetic molecularly imprinted nanobottles for specific adsorption and fast separation of luteolin. Chem Eng J 356:436–444. https://doi.org/10.1016/j.cej.2018.09.003
Ahmed A, Myers P, Zhang H (2014) Synthesis of nanospheres-on-microsphere silica with tunable shell morphology and mesoporosity for improved HPLC. Langmuir 30(41):12190–12199. https://doi.org/10.1021/la503015x
Wang H, Wu Y, Song H (2019) Synergistic effects of photonic crystal and gold nanostars for quantitative SERS detection of 3-phenoxybenzoic acid. Appl Surf Sci 476:587–593. https://doi.org/10.1016/j.apsusc.2019.01.061
Liu Y, Wu A, Hu J, Lin M, Wen M, Zhang X, Xu C, Hu X, Zhong J, Jiao L, **e Y, Zhang C, Yu X, Liang Y, Liu X (2015) Detection of 3-phenoxybenzoic acid in river water with a colloidal gold-based lateral flow immunoassay. Anal Biochem 483:7–11 https://doi.org/10.1016/j.ab.2015.04.022
El-Moghazy AY, Huo J, Amaly N, Vasylieva N, Hammock BD, Sun G (2020) An innovative nanobody-based electrochemical immunosensor using decorated nylon nanofibers for point-of-care monitoring of human exposure to pyrethroid insecticides. ACS Appl Mater Interfaces 12(5):6159–6168. https://doi.org/10.1021/acsami.9b16193
Funding
This work was financially supported by National Natural Science Foundation of China (31671934, 31801636), Shanghai Sailing Program (Grant No. 18YF1417300), and Shanghai Committee of Science and Technology (17391901500).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2687 kb)
Rights and permissions
About this article
Cite this article
Ye, T., Liu, A., Bai, L. et al. Core–satellite surface imprinting polymer-based pipette tip solid-phase extraction for the colorimetric determination of pyrethroid metabolite. Microchim Acta 187, 412 (2020). https://doi.org/10.1007/s00604-020-04394-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04394-5