Abstract
Restricted-access nanoparticles (RANPs) were prepared from bovine serum albumin by coacervation. They have an average sized of 311 nm. They were characterized and used to capture the β-blockers atenolol, metoprolol and propranolol from untreated biological samples. It is shown that both high protein affinity drugs (propranolol) and low protein affinity drugs (atenolol) could be rapidly extracted from plasma. This is revealed by kinetic and isothermal adsorption studies. On the other hand, almost all proteins from the sample were excluded. This demonstrates the efficiency of RANPs as restricted-access material. Sample preparation was carried out by solid phase microextraction using a probe obtained by the fixation of the RANPs at the end of a glass capillary. Atenolol (in concentrations from 100 to 1200 μg L−1), metoprolol (from 80 to 1000 μg L−1) and propranolol (from 15 to 200 μg L−1) were extracted from spiked plasma samples and analyzed by LC MS/MS without using a separation column. Correlation coefficients >0.99, good precision, accuracy, robustness, and lack of memory effects were observed for all of the analytes. The detection limits (at an S/N of 3) are 25.6, 14.6, and 3.8 μg L−1 for atenolol, metoprolol and propranolol, respectively. Ten samples can be simultaneously extracted within ∼15 min. Plasma samples of patients undergoing medical treatment were successfully analyzed with the method.

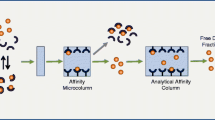
Schematic representation of a bovine serum albumin-based restricted access nanoparticle that exclude proteins from a human plasma sample but capture the small analytes.




Similar content being viewed by others
References
de Faria HD, Abrão LC de C, Santos MG et al (2017) New advances in restricted access materials for sample preparation: a review. Anal Chim Acta 959:43–65. https://doi.org/10.1016/j.aca.2016.12.047
Kataoka H (2018) Recent advances in online column-switching sample preparation. In: Reference module in chemistry, molecular sciences and chemical engineering. Elsevier, pp 1–30
Denadai M, Cass QB (2015) Simultaneous determination of fluoroquinolones in environmental water by liquid chromatography-tandem mass spectrometry with direct injection: a green approach. J Chromatogr A 1418:177–184. https://doi.org/10.1016/j.chroma.2015.09.066
Gomes RAB, Luccas PO, de Magalhães CS, de Figueiredo EC (2016) Evaluation of the pH influence on protein exclusion by restricted access carbon nanotubes coated with bovine serum albumin. J Mater Sci 51:7407–7414. https://doi.org/10.1007/s10853-016-9984-6
Shankar KR, Ameta RK, Singh M (2016) Preparation of BSA nanoparticles using aqueous urea at T = 308.15, 313.15 and 318.15 K as a function of temperature. J Mol Liq 216:808–813. https://doi.org/10.1016/j.molliq.2016.02.001
Tang QS, Chen DZ, Xue WQ et al (2011) Preparation and biodistribution of 188Re-labeled folate conjugated human serum albumin magnetic cisplatin nanoparticles (188Re-folate-CDDP/HSA MNPs) in vivo. Int J Nanomedicine 6:3077–3085. https://doi.org/10.2147/IJN.S24322
Elzoghby AO, Samy WM, Elgindy NA (2012) Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release 157:168–182. https://doi.org/10.1016/j.jconrel.2011.07.031
Nsubuga H, Basheer C, Haider MB, Bakdash R (2018) Sol-gel based biogenic silica composite as green nanosorbent for chemometric optimization of micro-solid-phase extraction of beta blockers. J Chromatogr A 1554:16–27. https://doi.org/10.1016/j.chroma.2018.04.044
Galisteo-González F, Molina-Bolívar JA (2014) Systematic study on the preparation of BSA nanoparticles. Colloid Surface B 123:286–292. https://doi.org/10.1016/j.colsurfb.2014.09.028
Barbosa AF, Barbosa VMP, Bettini J, Luccas PO, Figueiredo EC (2015) Restricted access carbon nanotubes for direct extraction of cadmium from human serum samples followed by atomic absorption spectrometry analysis. Talanta 131:213–220. https://doi.org/10.1016/j.talanta.2014.07.051
dos Santos RC, Kakazu AK, Santos MG, Belinelli Silva FA, Figueiredo EC (2017) Characterization and application of restricted access carbon nanotubes in online extraction of anticonvulsant drugs from plasma samples followed by liquid chromatography analysis. J Chromatogr B 1054:50–56. https://doi.org/10.1016/j.jchromb.2017.02.025
Silva FAB, Chagas-Silva FA, Florenzano FH, Pissetti FL (2016) Poly(dimethylsiloxane) and poly[vinyltrimethoxysilane-co-2-(dimethylamino) ethyl methacrylate] based cross-linked oroganic-inorganic hybrid adsorbent for copper(II) removal from aqueous solutions. J Braz Chem Soc 27:2181–2191. https://doi.org/10.5935/0103-5053.20160110
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10. https://doi.org/10.1016/j.cej.2009.09.013
Boscari CN, Mazzuia GR, Wisniewski C, Borges KB, Figueiredo EC (2017) Molecularly imprinted probe for solid-phase extraction of hippuric and 4-methylhippuric acids directly from human urine samples followed by MEKC analysis. Electrophoresis 38:1083–1090. https://doi.org/10.1002/elps.201600382
FDA - U.S. Department of Health and Human Services (2013) Guidance for industry: bioanalytical method validation. 34. http://www.labcompliance.de/documents/FDA/FDA-Others/Laboratory/f-507-bioanalytical-4252fnl.pdf
Rahimnejad M, Najafpour G, Bakeri G (2012) Investigation and modeling effective parameters influencing the size of BSA protein nanoparticles as colloidal carrier. Colloid Surface A 412:96–100. https://doi.org/10.1016/j.colsurfa.2012.07.022
Menezes ML, Fèlix G (1998) On line extraction and separation of bendiocarb, methomyl, methylparathion, and pentachlorophenol pesticides from raw milk. J Liq Chromatogr Relat Technol 21:2863–2871. https://doi.org/10.1080/10826079808003449
Bronze-Uhle ES, Costa BC, **menes VF, Lisboa-Filho PN (2017) Synthetic nanoparticles of bovine serum albumin with entrapped salicylic acid. Nanotechnol Sci Appl 10:11–21. https://doi.org/10.2147/NSA.S117018
Kumar KV, Sivanesan S (2006) Pseudo second order kinetics and pseudo isotherms for malachite green onto activated carbon: comparison of linear and non-linear regression methods. J Hazard Mater 136:721–726. https://doi.org/10.1016/j.jhazmat.2006.01.003
Liu Y, Liu Y-J (2008) Biosorption isotherms, kinetics and thermodynamics. Sep Purif Technol 61:229–242. https://doi.org/10.1016/j.seppur.2007.10.002
Kufleitner J, Wagner S, Worek F, von Briesen H, Kreuter J (2010) Adsorption of obidoxime onto human serum albumin nanoparticles: drug loading, particle size and drug release. J Microencapsul 27:506–513. https://doi.org/10.3109/02652041003681406
Figueiredo EC, Sparrapan R, Sanvido GB, Santos MG, Zezzi Arruda MA, Eberlin MN (2011) Quantitation of drugs via molecularly imprinted polymer solid phase extraction and electrospray ionization mass spectrometry: benzodiazepines in human plasma. Analyst 136:3753–3757. https://doi.org/10.1039/c1an15198c
Benfield P, Clissold SP, Brogden RN (1986) Metoprolol: an updated review of its Pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy, in hypertension, Ischaemic heart disease and related cardiovascular disorders. Drugs 31:376–429. https://doi.org/10.2165/00003495-198631050-00002
Leonetti G, Terzoli L, Bianchini C, Sala C, Zanchetti A (1980) Time-course of the anti-hypertensive action of atenolol: comparison of response to first dose and to maintained oral administration. Eur J Clin Pharmacol 18:365–374. https://doi.org/10.1007/BF00636787
Bengtsson C, Johnson G, Regdh CG (1975) Plasma levels and effects of metoprolol on blood pressure and heart rate in hypertensive patients after an acute dose and between two doses during long-term treatment. Clin Pharmacol Ther 17:400–408. https://doi.org/10.1002/cpt1975174400
Mansur AP, Avakian SD, Paula RS, Donzella H, Santos SRCJ, Ramires JAF (1998) Pharmacokinetics and pharmacodynamics of propranolol in hypertensive patients after sublingual administration: systemic availability. Brazilian J Med Biol Res 31:691–696. https://doi.org/10.1590/S0100-879X1998000500014
Fan W, He M, You L, Zhu X, Chen B, Hu B (2016) Water-compatible graphene oxide/molecularly imprinted polymer coated stir bar sorptive extraction of propranolol from urine samples followed by high performance liquid chromatography-ultraviolet detection. J Chromatogr A 1443:1–9. https://doi.org/10.1016/j.chroma.2016.03.017
Gorbani Y, Yılmaz H, Basan H (2017) Spectrofluorimetric determination of atenolol from human urine using high-affinity molecularly imprinted solid-phase extraction sorbent. Luminescence 32:1391–1397. https://doi.org/10.1002/bio.3335
Ensafi AA, Kazemifard N, Rezaei B (2017) Development of a nano plastic antibody for determination of propranolol using CdTe quantum dots. Sensor Actuat B-Chem 252:846–853. https://doi.org/10.1016/j.snb.2017.06.078
Kim HM, Park JH, Long NP, Kim DD, Kwon SW (2019) Simultaneous determination of cardiovascular drugs in dried blood spot by liquid chromatography-tandem mass spectrometry. J Food Drug Anal:1–9 (in press). https://doi.org/10.1016/j.jfda.2019.06.001
Elgawish MS, Mostafa SM, Elshanawane AA (2011) Simple and rapid HPLC method for simultaneous determination of atenolol and chlorthalidone in spiked human plasma. Saudi Pharm J 19:43–49. https://doi.org/10.1016/j.jsps.2010.10.003
Renkecz T, Ceolin G, Horváth V (2011) Selective solid phase extraction of propranolol on multiwell membrane filter plates modified with molecularly imprinted polymer. Analyst 136:2175–2182. https://doi.org/10.1039/c0an00906g
Jamshidi S, Rofouei MK, Thorsen G (2019) Using magnetic core-shell nanoparticles coated with an ionic liquid dispersion assisted by effervescence powder for the micro-solid-phase extraction of four beta blockers from human plasma by ultra high performance liquid chromatography with mass spectrom. J Sep Sci 42:698–705. https://doi.org/10.1002/jssc.201800834
Mabrouk MM, Hammad SF, El-Malla SF, Elshenawy EA (2019) Green micellar HPLC-fluorescence method for simultaneous determination of metoprolol and amlodipine in their combined dosage form: application on metoprolol in spiked human plasma. Microchem J 147:635–642. https://doi.org/10.1016/j.microc.2019.03.084
Acknowledgements
The authors thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Belo Horizonte, Brazil) [projects CDS-APQ-00638-17 and CDS-PPM-00144-15]; the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasília, Brazil) [project 427365/2018-0]; and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Restricted access nanoparticles (RANPs) of bovine serum albumin were synthesized.
2. RANPs efficiently excluded proteins from biological fluids.
3. Drugs with high and low protein binding rates were retained by the RANPs.
4. RANPs probes were useful, simple, and reproducible for solid phase microextraction.
5. The method was validated and used to determine β-blockers in human plasma samples.
Electronic supplementary material
ESM 1
(DOCX 37.0 kb)
Rights and permissions
About this article
Cite this article
Rosa, M.A., De Faria, H.D., Carvalho, D.T. et al. Biological sample preparation by using restricted-access nanoparticles prepared from bovine serum albumin: application to liquid chromatographic determination of β-blockers. Microchim Acta 186, 647 (2019). https://doi.org/10.1007/s00604-019-3774-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3774-1