Abstract
We report on the application of an automated and easy-to-use device to directly measure the immunoreactions between adda-specific monoclonal antibodies and microcystins. The antibodies were immobilized on a gold electrode whose surface was modified first with polytyramine and then with gold nanoparticles. The immunoreaction leads to a change in the capacitance of the system. Under optimum conditions, the sensor is capable of performing stable regeneration-assay cycles and has a low detection limit at a concentration of 0.01 pM level of microcystin-leucine-arginine (MC-LR). The surface of the biosensor can be regenerated with pH 2.5 glycine buffer which dissociates the antibody-antigen complex. The biosensor was used to monitor the production of microcystins during batch cultivation of Microcystis aeruginosa (isolated from ponds in Botswana). Liquid chromatography coupled to MS/MS detection was used to identify three variants, viz. MC-LR (995.6 Da), DmMC-LR (981.2 Da) and MC-LA (910.5 Da).

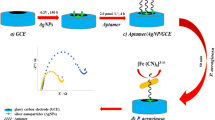
A capacitive immunosensor was fabricated by immobilizing monoclonal antibodies on a polytyramine-gold nanoparticle layer. The immunosensor was used to quantify microcystins produced by Microcystis aeruginosa; MC-LR, DmMC-LR and MC-LA, and further identified by LC- MS/MS. The results show that cumulative determination of microcystin variants is possible with this immunosensor.







Similar content being viewed by others
References
Antoniou MG, de la Cruz AA, Dionysiou DD (2005) Cyanotoxins: new generation of water contaminants. J Environ Eng 13(9):1239–1243
Namikoshi M, Sivonen K, Evans WR, Sun F, Carmichael WW, Rinehart KL (1992) Isolation and structures of microcystins from a cyanobacterial water bloom (Finland). Toxicon 30(11):1473–1479
Fischer WJ, Garthwaite I, Miles CO, Ross KM, Aggen JB, Chamberlin AR, Towers NR, Dietrich DR (2001) Congener-independent immunoassay for microcystins and nodularins. Environ Sci Technol 35:4849–4856
Dietrich DR, Fischer A, Michel C, Hoeger SJ (2008) Toxin mixture in cyanobacterial blooms—a critical comparison of reality with current procedures employed in human health risk assessment. In: Hudnell KH (Ed) Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Adv Exp Med Biol 619:885–912
World Health Organization (WHO) (1998) Guidelines for drinking water quality, addendum to vol.1, 2nd edn. World Health Organization, Geneva
Morais S, Tamarit-Lopez J, Puchades R, Maquieira A (2010) Determination of microcystins in river waters using microsensor arrays on disk. Environ Sci Technol 44:9024–9029
Long F, He M, Zhu AN, Shi HC (2009) Portable optical immunosensor for highly sensitive detection of microcystin-LR in water samples. Biosens Bioelectron 24:2346–2351
Herranz S, Marazuela MD, Moreno-Bondi MC (2012) Automated portable array biosensor for multisample microcystin analysis in freshwater samples. Biosens Bioelectron 33:50–55
Zeck A, Weller MG, Bursill D, Niessner R (2001) Generic microcystin immunoassay based on monoclonal antibodies against Adda. Analyst 126(11):2002–2007
Gambaro A, Barbaro E, Zangrando R, Barbante C (2012) Simultaneous quantification of microcystins and nodularin in aerosol samples using high-performance liquid chromatography/negative electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 26:1497–1506
Erlandsson D, Teeparuksapun K, Mattiasson B, Hedström M (2014) Automated flow-injection immunosensor based on current pulse capacitive measurements. Sensors Actuators B Chem 190:295–304
Teeparuksapun K, Hedström M, Wong EY, Tang S, Hewlett IK, Mattiasson B (2010) Ultrasensitive detection of HIV-p24 antigen using nanofunctionalised surfaces in a capacitive immunosensor. Anal Chem 82(20):8406–8411
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Komárek J, Komárkova J (2002) Review of the European Microcystis-morphospecies (Cyanoprokaryotes) from nature. Czech Phycol Olomouc 2:1–24
Phelan RR, Downing TG (2007) Optimization of laboratory scale production and purification of microcystin-LR from pure cultures of Microcystis aeruginosa. Afr J Biotechnol 6(21):2451–2457
Black K, Yilmaz M, Phlips EJ (2011) Growth and toxin production of Microcystis aeruginosa PCC 7806 (Kutzing) Lemmerman at elevated salt concentrations. J Environ Prot 2:669–674
Labib M, Hedström M, Amin M, Mattiasson B (2009) A capacitance immunosensor for detection of cholera toxin. Anal Chim Acta 634(2):255–261
Teeparuksapun K, Kanatharana P, Limbut W, Thammakhet C, Asawatreratanakul P, Mattiasson B, Wongkittisuksa B, Limsakul C, Thavarungkul P (2009) Disposable electrodes for capacitive immunosensor. Electroanalysis 21(9):1066–1074
Hedström M, Galaev IY, Mattiasson B (2005) Continuous measurements of a binding reaction using a capacitive biosensor. Biosens Bioelectron 21:41–48
Mattiasson B, Teeparuksapun K, Hedström M (2009) Immunochemical binding assays for detection and quantification of trace impurities in biotechnological production. Trends Biotechnol 28(1):20–27
Dietrich DR, Hoeger SJ (2005) Guidance values for microcystins in water and cyanobacterial supplement products (blue–green algal supplements): a reasonable or misguided approach? Toxicol Appl Pharmacol 203:273–289
**arrón JM, Yáñez-Sedeño P, González-Cortés A (2008) Gold nanoparticle-based electrochemical biosensors. Electrochim Acta 53:5848–5866
Losic D, Martin C, Thissen H, Voelcker NH (2005) Ultrathin polytyramine films by electropolymerisation on highly doped p-type silicon electrodes. Surf Sci 584:245–257
Queirós RB, Noronha JP, Marques PVS, Fernandes JS, Sales MGF (2012) Determination of microcystin-LR in waters in the subnanomolar range by sol–gel imprinted polymers on solid contact electrodes. Analyst 137(10):2437–2444
Tian J, Zhao H, Zhao H, Quan X (2012) Photoelectrochemical immunoassay for microcystin-LR based on a fluoride-doped tin oxide glass electrode modified with a Cds-graphene composite. Microchim Acta 179:163–170
Sun X, Guan L, Shi H, Ji J, Zhang Y, Li Z (2013) Determination of microcystin-LR with glassy carbon impedimetric immunoelectrode modified with an ionic liquid and multiwalled carbon nanotubes. Microchim Acta 180:75–83
Tong P, Tang S, He Y, Shao Y, Zhang L, Chen G (2011) Label-free immunosensing of microcystin-LR using gold electrode modified with gold nanoparticles. Microchim Acta 173:299–305
Msagati TAM, Siame BA, Shushu DD (2006) Evaluation of methods for the isolation, detection and quantification of cyanobacterial hepatotoxins. Aquat Toxicol 78:382–397
Hartwell SK, Grudpan K (2010) Flow based immune/bioassays and trends in micro-immuno/biosensors. Microchim Acta 169:201–220
Lawrence JF, Niedzwiadek B, Menard C, Lau BP, Lewis D, Kuper-Goodman T, Carbone S, Holmes C (2001) Comparison of liquid chromatography/mass spectrometry, ELISA, and phosphatase assay for the determination of microcystins in blue-green algae products. J AOAC Int 84(4):1035–1044
Wang J, Pang X, Ge F, Ma Z (2007) An ultra-performance liquid chromatography-tandem mass spectrometry method for determination of microcystins occurrence in surface water in Zhejiang Province, China. Toxicon 49:1120–1128
Silva-Stenico ME, Neto RM, Alves IR, Moraes LAB, Shishido TK, Fiore MF (2009) Hepatotoxin microcystin-LR extraction optimization. J Braz Chem Soc 4:535–542
Allis O, Dauphard J, Hamilton B, Shuilleabhain AN, Lehane M, James KJ, Furey A (2007) Liquid chromatography–Tandem mass spectrometry application, for the determination of extracellular hepatotoxins in Irish lake and drinking waters. Anal Chem 79:3436–3447
Oehrle SA, Southwell B, Westrick J (2010) Detection of various freshwater cyanobacterial toxins using ultra-performance liquid chromatography tandem mass spectrometry. Toxicon 55:965–972
Yuan M, Namikoshi M, Otsuki A, Sivonen K (1998) Effect of amino acid side-chain on fragmentation of cyclic peptide ions: differences of electrospray ionization/collision-induced decomposition mass spectra of toxic heptapeptide micorcystin containing ADMAdda instead of Adda. Eur J Mass Spectrom 4:287–298
Reverté L, Garibo D, Flores C, Diogène J, Caixach J, Campàs M (2013) Magnetic particle-based enzyme assays and immunoassays for microcystins: from colorimetric to electrochemical detection. Environ Sci Technol 47:471–478
Hiller S, Krock B, Cembella A, Luckas B (2007) Rapid detection of cyanobacterial toxins in precursor ion mode by liquid chromatography tandem mass spectrometry. J Mass Spectrom 42:1238–1250
Acknowledgments
Botswana International University of Science and Technology is gratefully acknowledged for the financial support. The Research Council of Sweden (VR) also supported a part of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lebogang, L., Mattiasson, B. & Hedström, M. Capacitive sensing of microcystin variants of Microcystis aeruginosa using a gold immunoelectrode modified with antibodies, gold nanoparticles and polytyramine. Microchim Acta 181, 1009–1017 (2014). https://doi.org/10.1007/s00604-014-1199-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1199-4