Abstract
Purpose
Advances in primary lung cancer drug therapy have extended patients’ survival, including patients with stage IV disease. This study assessed the safety and effectiveness of salvage surgery following tyrosine kinase inhibitor (TKI) or immune checkpoint inhibitor (ICI) therapy in primary lung cancer.
Methods
A retrospective chart review was conducted of 2050 primary lung cancer surgeries performed at our institution between 2012 and 2022. The study included patients who underwent salvage surgery for unresectable lesions that became resectable or localized residual lesions after treatment. We investigated patients’ clinicopathological characteristics, therapeutic responses, and survival outcomes.
Results
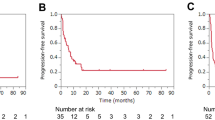
We identified eight cases of salvage surgery after TKI treatment and eight cases after ICI treatment. Five patients experienced early recurrence after surgery; however, the long-term outcome in the post-TKI group was favorable, with a median overall survival (OS) of 66 (range: 28–80) months. Postoperative recurrence was confined to local lymph node recurrence in one patient in the post-ICI group. Despite the relatively short observation period, the long-term prognosis remained promising, with a median OS of 18.7 (range: 9.7–55.8) months.
Conclusions
Salvage surgery after TKI or ICI treatment can be safely performed, and the OS may be favorable.

Similar content being viewed by others
Abbreviations
- OS:
-
Overall survival
- RFS:
-
Recurrence-free survival
- TKI:
-
Tyrosine kinase inhibitor
- ICI:
-
Immune checkpoint inhibitor
- RECIST:
-
Response evaluation criteria in solid tumors
- EGFR-TKIs:
-
Epidermal growth factor receptor tyrosine kinase inhibitors
- ALK:
-
Anaplastic lymphoma kinase
- PR:
-
Partial response
- SD:
-
Stable disease
- CR:
-
Complete response
- pCR:
-
Pathological complete response
- MPR:
-
Major pathological response
References
Bauman JE, Mulligan MS, Martins RG, Kurland BF, Eaton KD, Wood DE. Salvage lung resection after definitive radiation(>59Gy) for non-small cell lung cancer: surgical and oncologic outcomes. Ann Thorac Surg. 2008;86:1632–8.
Kuzmik GA, Detterbeck FC, Decker RH, Boffa DJ, Wang Z, Oliva IB, et al. Pulmonary resections following prior definitive chemoradiation therapy are associated with acceptable survival. Eur J Cardiothorac Surg. 2013;44:e66-70.
Yang CF, Meyerhoff RR, Stephens SJ, Singhapricha T, Toomey CB, Anderson KL, et al. Long-term outcomes of lobectomy for non-small cell lung cancer after definitive radiation treatment. Ann Thorac Surg. 2015;99:1914–20.
Yang CJ, Gu L, Shah SA, Yerokun BA, D’Amico TA, Hartwig MG, et al. Long-term outcomes of surgical resection for stage IV non-small-cell lung cancer: a national analysis. Lung Cancer. 2018;115:75–83.
Jia J, Guo B, Yang Z, Liu Y, Ga L, **ng G, et al. Outcomes of local thoracic surgery in patients with stage IV non-small-cell lung cancer: a SEER-based analysis. Eur J Cancer. 2021;144:326–40.
Shimizu K, Ohtaki Y, Suzuki K, Date H, Yamashita M, Iizasa T, et al. Salvage surgery for non-small cell lung cancer after definitive radiotherapy. Ann Thorac Surg. 2021;112:862–73.
Ohtaki Y, Shimizu K, Suzuki H, Suzuki K, Tsuboi M, Mitsudomi T, et al. Salvage surgery for non-small cell lung cancer after tyrosine kinase inhibitor treatment. Lung Cancer. 2021;153:108–16.
Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine E, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 Study. J Clin Oncol. 2023;41:1992–8.
Brahmer JR, Lee JS, Ciuleanu TE, Caro RB, Nishio M, Urban L, et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in CheckMate 227. J Clin Oncol. 2023;41:1200–12.
Bott MJ, Cools-Lartigue J, Tan KS, Dycoco J, Bains MS, Downey RJ, et al. Safety and feasibility of lung resection after immunotherapy for metastatic or unresectable tumors. Ann Thorac Surg. 2018;106:178–83.
Baek J, Owen DH, Merritt RE, Shilo K, Otterson GA, D’Souza DM, et al. Minimally invasive lobectomy for residual primary tumors of advanced non-small-cell lung cancer after treatment with immune checkpoint inhibitors: case series and clinical considerations. Clin Lung Cancer. 2020;21:e265–9.
Bertolaccini L, Galetta D, Sedda G, de Marinis F, Spaggiari L. Safety analysis of salvage surgery for advanced stages or metastatic lung cancers. Thorac Cardiovasc Surg. 2022;70:273–6.
Smith A, Wali A, Montes A, Hadaki M, Harrison-Phipps K, Karapanagiotoue EM, et al. Salvage pulmonary resection in stages IIIb–IV lung cancer after treatment with immune checkpoint inhibitors case series and literature review. J Surg Oncol. 2022;125:290–8.
Beattie R, Furrer K, Dolan DP, Curioni-Fontecedro A, Lee DN, Frauenfelder T, et al. Two centres experience of lung cancer resection in patients with advanced non-small cell lung cancer upon treatment with immune checkpoint inhibitors: Safety and clinical outcomes. Eur J Cardiothorac Surg. 2021;60:1297–305.
Nagata S, Hamaji M, Ozasa H, Yamada Y, Ohsumi A, Date H. Salvage surgery after immune checkpoint inhibitors for advanced non-small cell lung cancer: potential association between immune-related adverse events and longer survival. Clin Lung Cancer. 2022;23:e321–4.
Ueno T, Yamashita M, Yamashita N, Uomoto M, Kawamata O, Sano Y, et al. Safety of salvage lung resection after immunotherapy for unresectable non-small cell lung cancer. Gen Thorac Cardiovasc Surg. 2022;70:812–7.
Imanishi N, Yoneda K, Taira A, Ichiki Y, Sato N, Hisaoka M, et al. Major pathologic response to alectinib in ALK-rearranged adenocarcinoma of the lung. Surg Case Rep. 2018;4:19. https://doi.org/10.1186/s40792-018-0430-7.
Chikaishi Y, Uramoto H, Oka S, Nagata S, Shimokawa H, So T, et al. Discrepancy between the clinical image and pathological findings of non-small cell lung cancer harboring an epidermal growth factor receptor gene mutation that was surgically resected after gefitinib treatment. Case Rep Oncol. 2014;7:126–31.
Nawashiro A, Tanaka F, Taira A, Shinohara S, Takenaka M, Kuroda K, et al. Salvage surgery following immuno-chemo-radiotherapy for advanced non-small cell lung cancer. Surg Case Rep. 2022;8:17.
Fujita Y, Take N, Shinohara S, Mori M, Kanayama M, Takenaka M, et al. Achievement of pathological complete response with Osimertinib for EGFR-mutated lung adenocarcinoma. General Thorac Cardiovasc Surg Cases. 2023;2:60.
Lin MW, Yu SL, Hsu YC, Chen YM, Lee YH, Hsiao YJ, et al. Salvage surgery for advanced lung adenocarcinoma after epidermal growth factor recepter tyrosine kinase inhibitor treatment. Ann Thorac Surg. 2023;116:111–9.
Chen YY, Yen YT, Lai WW, Huang WL, Chang CC, Tseng YL. Outcomes of salvage lung resections in advanced EGFR-mutant lung adenocarcinomas under EGFR TKIs. Thorac Cancer. 2021;12:2655–65.
**ong L, Li R, Sun J, Lou Y, Zhang W, Bai H, et al. Erlotinib as neoadjuvant therapy in stage IIIA (N2) EGFR mutation-positive non-small cell lung cancer: a prospective, single-arm, phase II study. Oncologist. 2019;24:157-e64.
Zhang Y, Fu F, Hu H, Wang S, Li Y, Hu H, et al. Gefitinib as neoadjuvant therapy for resectable stage II–IIIA non–small cell lung cancer: a phase II study. J Thorac Cardiovasc Surg. 2021;161:434-442.e2.
Zhong WZ, Chen KN, Chen C, Gu CD, Wang J, Yang XN, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non-small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37:2235–45.
Zhao Y, Cheng B, Chen Z, Li J, Liang H, Chen Y, et al. Toxicity profile of epidermal growth factor receptor tyrosine kinase inhibitors for patients with lung cancer: a systematic review and network meta-analysis. Crit Rev Oncol Hematol. 2021;160: 103305.
Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386:1973–85.
Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1413–22.
Provencio M, Serna-Blasco R, Nadal E, Insa A, García-Campelo MR, Rubio JC, et al. Overall survival and biomarker analysis of neoadjuvant nivolumab plus chemotherapy in operable stage IIIA non-small-cell lung cancer (NADIM phase II trial). J Clin Oncol. 2022;40:2924–33.
Cascone T, William WN Jr, Weissferdt A, Leung CH, Lin HY, Pataer A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med. 2021;27:504–14.
Acknowledgements
The authors wish to thank all of their colleagues in the Second Department of Surgery (Chest Surgery), Hospital of the University of Occupational and Environmental Health, Japan, for their helpful contributions to perioperative care. We would also like to thank Editage (www.editage.com) for the English language editing.
Funding
This work was not supported by any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
MT: Investigation, Methodology, Writing—original draft. FT: Conceptualization, Investigation, Supervision, Writing, review, and editing. KK: Investigation, Methodology, Data curation. TM: Investigation, Methodology, Data curation. KY: Investigation, Methodology, Data curation. MM: Investigation, Methodology, Data curation. MK: Investigation, Methodology, Data curation. AT: Supervision, Writing—review. TK: Supervision, Writing—review. AN: Investigation, Data curation. KK: Investigation, Supervision, Writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
FT received research grants from Boehringer Ingelheim Japan, Ono Pharmaceutical, Taiho Pharmaceutical, Eli Lilly Japan, and Chugai Pharmaceutical. FT also reports receiving consulting fees from AstraZeneca, Chugai Pharmaceutical, and Ono Pharmaceutical, as well as payment for lectures from MSD, Bristol-Myers Squibb, Boehringer Ingelheim Japan, Ono Pharmaceutical, Taiho Pharmaceutical, Eli Lilly Japan, AstraZeneca, Chugai Pharmaceutical, Kyowa-Kirin, Takeda Pharmaceutical, and Pfizer. The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meeting presentation: This manuscript was presented at the 31st Annual Meeting of the Asian Society for Cardiovascular and Thoracic Surgery held at BEXCO in Busan, Korea on June 1, 2023.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takenaka, M., Tanaka, F., Kajiyama, K. et al. Outcomes and pathologic response of primary lung cancer treated with tyrosine kinase inhibitor/immune checkpoint inhibitor before salvage surgery. Surg Today (2024). https://doi.org/10.1007/s00595-024-02811-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00595-024-02811-3