Abstract
The COronaVirus DISease 19 (COVID-19) is a pandemic infectious disease caused by the novel coronavirus Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS-CoV-2). Older age and presence of comorbidities, including diabetes, were shown to be associated with a more severe course and a higher fatality rate. Studies from the most affected countries, including China, United States and Italy, seem to indicate that prevalence of diabetes among patients affected by COVID-19 is not higher than that observed in the general population, thus suggesting that diabetes is not a risk factor for SARS-CoV-2 infection. However, a large body of evidence demonstrate that diabetes is a risk factor for disease progression towards critical illness, development of acute respiratory distress syndrome, need for mechanical ventilation or admission to intensive care unit, and ultimately death. The mechanisms underlying the relationship between COVID-19 and diabetes remain to be elucidated. In particular, it is still unresolved whether is diabetes per se, especially if poorly controlled, or rather the various comorbidities/complications associated with it that predispose patients with COVID-19 to a worse prognosis. In fact, conditions that cluster with diabetes in the context of the metabolic syndrome, such as obesity and hypertension, or complicate chronic hyperglycemia, such as cardiovascular disease and chronic kidney disease, have also been associated with poor prognosis in these individuals and the available studies have not consistently shown that diabetes predict disease severity independently of them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The COronaVirus DISease 19 (COVID-19) is an infectious disease caused by a novel coronavirus, the Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS-CoV-2) [1], belonging to the same family of viruses as the SARS-CoV and the Middle East Respiratory Syndrome CoronaVirus (MERS-CoV), which caused serious outbreaks in 2003 and 2012, respectively [2, 3]. The disease has originated in December 2019 in Wuhan, the capital of the Hubei province in China [1], and has rapidly spread around the globe as a pandemic [4].
Infection with SARS-CoV2 is characterized by a wide range of clinical presentations from asymptomatic, yet contagious forms to severe and potentially lethal illness. As other coronaviruses, SARS-CoV2 predominantly causes respiratory manifestations, including flulike symptoms and interstitial pneumonia, which may rapidly progress to acute respiratory distress syndrome (ARDS) requiring admission to intensive care unit (ICU) [5]. However, other organs are also affected, especially the heart, liver, and kidneys, and some patients eventually die of multi-organ failure [5]. Moreover, ageusia and anosmia are characteristic, usually reversible symptoms of COVID-19, though it is unclear whether these disturbances are related to damage of taste and olfactory neurons [5]. Other common manifestations of COVID-19 involve the hematopoietic, hemostatic, and immune systems and include lymphopenia and hypercoagulability, which correlate with disease severity [6]. Lymphopenia is associated with reduction in total T cells, CD4+ and CD8+ T cell subsets, B cells, and natural killer cells [7] and overproduction of several pro-inflammatory cytokines, which is often massive and may cause a “cytokine storm” [8]. Hypercoagulability is evidenced by a characteristic elevation of D-dimer and other fibrin degradation products and by prothrombin time prolongation [9]. Abnormalities in hemostasis may result in widespread thrombosis associated with microvascular injury in the lungs and other affected organs [10] and may even end-up with life-threatening disseminated intravascular coagulation [9].
A population-based study from Iceland has shown a lower incidence of SARS-CoV-2 infection in children and females compared to adolescents or adults and males [11]. Among symptomatic individuals hospitalized for COVID-19, older age and presence of comorbidities, including diabetes, obesity, hypertension, chronic obstructive respiratory disease (COPD), cardiovascular disease (CVD), chronic kidney disease (CKD), cancer, and immunodeficiency states, were shown to be associated with a more severe course and a higher fatality rate [12, 13]. This article reviews the existing literature on the role of diabetes as a risk factor for SARS-CoV-2 infection and outcomes, with particular reference to the data on the prevalence of diabetes among COVID-19 patients and its relationship with the severity of the disease. Moreover, the possible mechanisms linking diabetes to COVID-19 will be discussed, with a focus on the role of diabetes-associated comorbidities/complications.
Prevalence of diabetes among COVID-19 patients
Initial, relatively small studies from Wuhan and the Hubei province and also from other Chinese regions have shown variable prevalence rates of diabetes among patients hospitalized with COVID-19 (Supplemental Table 1), with differences likely reflecting the age of the sample, the hospital setting, and the geographical area (i.e., with high or low COVID-19 prevalence).
Larger studies from China provided more consistent prevalence rates (Table 1). In two multicenter, nationwide reports, diabetes was present in 7.4% of 1099 (median age 47 years) [14] and in 8.2% of 1, 590 (mean age, 48.9 years) [15] hospitalized individuals. Moreover, of 7337 individuals admitted to nineteen hospitals in the Hubei province (median age 54 years), 952 (13.0%) had type 2 diabetes [16], whereas a report of the Chinese Center for Disease Control and Prevention (China CDC), which however included also non-hospitalized individuals, showed a lower prevalence of diabetes (5.3%) among 44,672 confirmed COVID-19 cases through February 11, 2020 [17]. Several meta-analyses including Chinese COVID-19 patients confirmed a prevalence of diabetes of approximately 8–10% [18,19,20,21,22,23], i.e., not higher, if anything, than that in the general population, which was 12.9% in 2013 among people aged 40–59 years [24]. Moreover, prevalence was 11.2% (9.8% after adjusting for heterogeneity) in a meta-analysis including also two studies from US and one study from France [25] and as high as 23.8% in another meta-analysis in which more than half of patients were from a US study [13] (Table 2).
Regarding surveys from outside China (Table 3), a single-center study from Padua, Italy, showed that, among 146 hospitalized patients (mean age 65.3 years), prevalence of diabetes was 8.9% [21], again not higher than in the coeval general population from the same region (11.0%) [26]. Another single-center Italian survey from Milan reported a diabetes prevalence of 14.9% among 410 hospitalized individuals with COVID-19 (median age 65 years) [27]. Conversely, prevalence of diabetes was higher among 1339 COVID-19 from seven hospitals in Madrid, Spain (mean age 69.1 years), as compared with 13,390 matched controls (27.2% vs 20.3%; crude odds ratio, OR, 1.50 [95% confidence interval, CI, 1.30–1.73]) [28]. Prevalence rates were even higher in US patients hospitalized with COVID-19, ranging from 22.6 to 37.2% [29,30,31,32,33,34]. These figures are much higher than those reported in the general US, i.e., 13.0%, and even higher than those observed among US individuals aged 45–64 years, i.e., 17.5%, though in most studies the median age was close to upper limit of this range [35]. However, among 7162 COVID-19 cases with complete information reported to the US Centers for Disease Control and Prevention (CDC) and including also non-hospitalized patients, the overall diabetes prevalence was 10.9% [36].
Altogether, data from all over the world indicate that prevalence of diabetes among patients affected by COVID-19 is not higher than that observed in the general population, especially if considering that most of the existing reports are from hospitalized patients and, hence, may suffer from a selection/referral bias that may result in an increased prevalence of diabetes by excluding less severe, non-hospitalized individuals. Thus, available data suggest that diabetes is not a risk factor for SARS-CoV-2 infection.
Impact of diabetes on COVID-19 course and outcome
Several studies and meta-analyses have examined the impact of diabetes on COVID-19 severity, defined as need versus no need for hospitalization, critical versus non-critical illness, progression versus non-progression, ARDS versus non-ARDS, requirement versus no requirement of ICU admission or mechanical ventilation, fatal versus non-fatal disease, or occurrence versus non-occurrence of a composite outcome combining them.
The initial studies from China almost consistently reported a higher prevalence of diabetes among patients with severe versus non-severe disease, though differences were not always significant, likely due to the relatively small sample size (Supplemental Table 1). Larger studies provided more robust findings (Table 1). The two nationwide studies by Guan et al. reported a higher prevalence of diabetes among patients with severe versus non-severe disease (16.2% vs 5.7% and 17.7% vs 6.4%, respectively) and among those who reached versus those who did not reach a composite outcome (admission to ICU, invasive ventilation or death; 26.9% vs 6.1% and 23.7% vs 6.8%, respectively) [14, 15]. Likewise, Zhu et al. reported a higher prevalence of type 2 diabetes in patients with versus without ARDS (25.9% vs 6.9%) and in those who died versus those who survived (29.8% vs 12.4%); moreover, ARDS and mortality rates were higher in diabetic versus non-diabetic individuals (16.9% vs 7.2%, p < 0.001, and 7.8% vs 2.7%, p < 0.001, respectively) [16]. In a China CDC report, diabetes prevalence rose from 5.3% in the whole cohort to 19.7% among non-survivors and case fatality rate rose from 2.3% in the whole cohort to 7.3% among diabetic individuals [17].
Similar results were obtained also outside China (Table 3). The most recent nationwide report from the National Institute of Health of Italy showed a diabetes prevalence of 29.8% among 3857 COVID-19 patients (median age 82 years) who deceased through July 9, 2020 [37]. In a whole population study from UK, of 23,804 COVID-19 related in-hospital deaths, one third occurred in people with diabetes, i.e., 7466 (31.4%) with type 2 and 365 (1.5%) with type 1, and crude mortality rates and the adjusted odds of dying in hospital with COVID-19 were much higher in patients with than in those without diabetes [38]. Among COVID-19 cases reported to the US CDC, prevalence of diabetes rose from 6.4% in non-hospitalized patients, to 24.2% and 32.4% among those admitted to non-ICUs and ICUs, respectively [36]. Likewise, in a single-center study, prevalence of diabetes increased from 26.0% among individuals in the emergency room (ER), to 37.8% and 42.8% among non-ICU and ICU patients, respectively [32]. Other US surveys reported a higher diabetes prevalence in patients admitted to hospital versus those not (34.7% vs 9.7%) and in those critical versus not (39.3% vs 32.0%) [29], in patients admitted to ICU versus those not (39.8% vs 27.7%) [33], and in those who deceased versus those not (40.5% vs 25.6%) [30].
Several meta-analyses examined the association between diabetes and disease severity and expressed results as OR, risk ratio (RR), or rate ratio (Table 2). Wang et al. [18], Zheng et al. [22], and Tian et al. [13], but not Yang et al. [19], showed that risk of diabetes was higher in severe/deceased than in non-severe/survived patients, i.e., OR, 2.47 (95% CI, 1.67–3.66), 3.68 (2.68–5.03), 1.97 (1.67–2.31), and 2.07 (0.89–4.82), respectively. Consistent with Yang et al., Jain et al. found that diabetes was associated with severe disease only with borderline significance (pooled OR, 3.12 [95% CI, 1.0–9.75], p = 0.05) and was not associated with ICU admission (2.72 [0.70–10.6], p = 0.15) [12], whereas Kumar et al. reported a pooled OR of 2.75 (95% CI, 2.09–3.62), p < 0.01, for severe disease, of 1.90 (1.37–2.64), p < 0.01, for death, and of 2.49 (1.98–3.14), p < 0.01, for the combined endpoint [25]. Moreover, Fadini et al. reported a pooled rate ratio of diabetes among those with more severe versus less severe illness of 2.26 (95% CI, 1.47–3.49) [21], whereas Li et al. showed an about twofold higher proportion of diabetes in ICU/severe cases than in their non-ICU/non-severe counterparts (RR, 2.21 [95% CI, 0.88–5.57]) [20]. Huang et al. showed that diabetes was associated with a composite poor outcome (RR, 2.38 [95% CI, 1.88–3.03], p < 0.001) and its components [39].
The data reviewed above clearly indicate that diabetes is a risk factor for disease progression towards critical illness, development of ARDS, need for mechanical ventilation/ICU, and ultimately death, though the mechanisms underlying this relationship remain to be elucidated.
Mechanisms linking diabetes to COVID-19
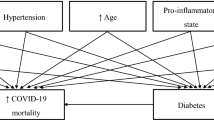
Several mechanisms have been claimed for explaining the exacerbating effect of diabetes on COVID-19 (Fig. 1). These mechanisms include those directly related to hyperglycemia and the associated imbalances in pathways involved in virus entry into the cell as well as in the immune and inflammatory response. Alternatively, the effect of diabetes may be mediated by diabetes-related comorbidities/complications that have also been associated with poor prognosis.
Glycemic control
Whether poor glycemic control is a risk factor for disease progression in COVID-19 patients is still a matter of debate. In fact, a retrospective, multicenter survey from the Hubei Province in China showed that patients with well-controlled blood glucose levels had lower rates of death (1.1% vs 11.0%; hazard ratio, HR, adjusted for age and gender and hospital sites, 0.10 [95% CI, 0.03–0.32], p < 0.001), ARDS (7.1% vs 21.4%; 0.32 [0.20–0.51], p < 0.001), and other complications, compared with individuals with poorly controlled blood glucose levels [16]. Likewise, a whole population study from UK showed that risk of death, adjusted for region of residence and duration of diagnosed diabetes, increased significantly for a hemoglobin (Hb) A1c ≥ 86 and ≥ 59 (and < 48) mmol/mol in individuals with type 1 and 2 diabetes, respectively [38]. Conversely, the CORONADO study, a prospective, multicenter survey from France, showed that long-term glycemic control was not associated with a composite outcome including mechanical ventilation and/or death, either in the univariable or in the multivariable analysis [40].
However, in this scenario, it should be considered also the possible diabetogenic effect of SARS-CoV2 due to its direct action on key metabolic organs, including the β-cell, and resulting in new-onset hyperglycemia or sudden deterioration of pre-existing diabetes, beyond the well-recognized stress response associated with severe illness [41]. Three recent reports showed that fasting blood glucose at admission, irrespective of previous diagnosis of diabetes, was an independent predictor of critical illness [42], death [43], or poor outcome [44] in patients hospitalized with COVID-19.
The practical recommendations for the management of diabetes in patients with COVID-19 outline the importance of achieving optimal glycemic control and point out potential metabolically interfering effects of metformin, sodium-glucose-co-transporter 2 inhibitors, and glucagon-like peptide-1 receptor agonists related to dehydration [45]. In addition, medications commonly used in diabetic patients, including anti-hyperglycemic, lipid-lowering, and anti-hypertensive drugs, have been implicated in COVID-19 onset and progression by virtue of their pleiotropic effects, especially on inflammation. However, to date, there is no evidence that these agents play a major role by either favoring disease progression or protecting from development of critical illness. The CORONADO study showed no association with disease severity of routine therapies for people with diabetes, including blockers of the renin–angiotensin system (RAS) and inhibitors of dipeptidyl peptidase-4 (DPP4) [40], and an Italian case–control study reported no effect of treatment with DPP4 inhibitors on hospitalization for COVID-19 [46].
Facilitation of virus entry into the cell
It has been suggested that diabetes may detrimentally impact the course of the disease through its effects on receptors that mediate virus entry into the cell. Coronavirus receptor proteins include the angiotensin-converting enzyme 2 (ACE2) and DPP4, which are both involved in the regulation of several physiological processes, including glucose metabolism, and are modulated by hyperglycemia and pharmacological treatments commonly used in diabetic individuals [47]. Moreover, both receptors exist as a transmembrane and a soluble form, with the latter potentially serving as decoy receptor, which binds and sequesters circulating virus particles [47]. As DPP4 is a receptor for MERS-CoV [48], but not for SARS-Cov-2 [49], ACE2 has gained most of the attention. A large body of evidence has indicated that treatment with RAS blockers, which increase ACE2 expression, is not associated with either COVID-19 diagnosis or poorer disease outcomes [50,51,52], thus arguing against the hypothesis that the exacerbating effect of diabetes (as well as of hypertension, CVD, and CKD) on COVID-19 is mediated through ACE2 upregulation associated with pharmacological RAS blockade. Therefore, discontinuation of these agents is not recommended in COVID-19 patients in order to maintain their anti-hypertensive and cardioprotective effects [53]. Indeed, it has been postulated that binding of SARS-Cov-2 to ACE2, by reducing the expression and/or the activity of this receptor, enhances the vasoconstrictor and pro-inflammatory/pro-oxidant activity of angiotensin II, thus increasing the risk for acute lung injury (ALI), and that RAS blockade may help mitigate these deleterious effects of angiotensin II [55]. However, though this concept is consistent with the finding that ACE2 protects from ALI [54] and with a few studies and a meta-analysis reporting a benefit with RAS blockers [55,56,57], current guidelines do not recommend initiating treatment with these agents in COVID-19 patients unless clinically indicated [53].
In addition to RAS activation, diabetes and related comorbidities are also associated with elevated levels of plasmin (ogen), a protease that cleaves the S protein of SARS-CoV2, thus favoring virus binding to ACE2 and entry into the cell; moreover, fibrin breakdown by plasmin leads to increased levels of D-dimer and other fibrin degradation products, which are characteristic features of severe illness [58].
Impaired immune and inflammatory response
Diabetes may also favor the onset and progression of SARS-CoV2 infection by impairing the adaptive immune response to the virus, while enhancing the innate immune system inflammatory reaction. Diabetes has long been recognized as a risk factor for morbidity and mortality from various types of infections, including those caused by respiratory viruses [59]. In addition, diabetes is also known to be accompanied by a chronic pro-inflammatory and pro-coagulant state, albeit of low grade, which characterizes also the associated comorbidities/complications [60]. Increased susceptibility to infections has been related to several immune defects, including blunted anti-viral interferon-γ response, delayed activation of CD4+ cells with shift toward Th17 responses, and diminished regulatory T cells, which all contribute to hyperinflammation [61]. In patients with COVID-19, immune, inflammatory and coagulation abnormalities were found to be significantly more pronounced in diabetic than in non-diabetic individuals [62], independently of other comorbidities [63], and to correlate with glycemic control [64]. As these abnormalities were shown to predict disease severity and adverse outcomes, it was suggested that they may be responsible for the exacerbating effect of diabetes (and related comorbidities) [59].
Diabetes-related comorbidities
The negative impact of diabetes on COVID-19 may not (or not only) be related to hyperglycemia per se, but rather to the comorbidities that frequently associate with it. These comorbidities include obesity and hypertension, which usually cluster with type 2 diabetes in the context of the metabolic syndrome, as well as CVD and CKD, which are common and severe complications of chronic hyperglycemia.
Studies in COVID-19 patients have confirmed that all these comorbidities are more frequent in those with than in those without diabetes [16, 62]. In addition, studies have provided evidence that, just like diabetes, these comorbidities are highly prevalent in COVID-19 patients. In particular, hypertension has been shown to be the most prevalent comorbidity, followed by diabetes and, in non-Asian patients, obesity; in addition, CKD and CVD, including coronary heart disease (CHD), heart failure, and cerebrovascular disease, were also frequently observed in patients with SARS-CoV2 infection. Among COVID-19 cases reported to the China CDC, prevalence of hypertension and CVD were 12.8% and 4.2%, respectively [17], whereas two nationwide surveys on hospitalized Chinese patients with COVID-19 showed prevalence rates of hypertension of 15.0% and 16.9%, of CHD/CVD of 2.5% and 3.7%, of cerebrovascular disease of 1.4% and 1.9%, and of CKD of 0.7% and 1.3%, respectively [14, 15]. Among 7162 COVID-19 cases with complete information reported to the US CDC, CVD and CKD were found in 9.0% and 3.0% of patients, respectively [36], whereas prevalence of hypertension, obesity, and CVD was 49.7%, 48.3%, and 27.8%, respectively, among 1482 individuals hospitalized with COVID-19 in fourteen US states [34]. Moreover, in a case series of 5700 patients from the New York City area, prevalence of hypertension, obesity, CHD, heart failure, CKD, and end-stage renal disease (ESRD) were 56.6%, 41.7%, 11.1%, 6.9%, 5.0%, and 3.5%, respectively [30].
As for diabetes, all these comorbidities were found to be more prevalent in patients with more severe or fatal illness than in those with less severe and non-fatal disease in virtually all studies of adequate sample size conducted on Chinese, Italian, or US COVID-19 patients. For instance, among cases reported to the China CDC, case fatality rates were 6.0% and 10.5% in individuals with hypertension and CVD, respectively, versus 2.3% in the whole cohort [17], whereas two nationwide Chinese surveys reported higher prevalence of hypertension, CHD/CVD, cerebrovascular disease, and CKD in patients with severe versus non-severe disease and in those who reached versus those who did not reach a composite outcome [14, 15]. Prevalence of hypertension, CHD, atrial fibrillation, heart failure, stroke, CKD, and ESRD were 66.2%, 27.7%, 23.0%, 15.8%, 10.4%, 20.3%, and 2.0%, respectively, among COVID-19 Italian patients who deceased through July 9, 2020 [37]. Moreover, in a single-center study from New York, prevalence rates of hypertension, CHD, heart failure, stroke, and CKD increased according to the setting, from 50.7%, 10.7%, 7.3%, 4.7%, and 8.0%, respectively, among ER patients, to 59.8%, 14.0%, 10.9%, 8.6% and 16.0%, respectively, among non-ICU patients, and 66.9%, 12.3%, 10.2%, 8.1% and 11.4%, respectively, among ICU patients [32]. Finally, several meta-analyses provided robust evidence of a higher prevalence of hypertension and CVD [12, 13, 18,19,20, 22], but also of cerebrovascular disease and CKD [13, 18, 22], in patients with than in those without severe/fatal disease.
However, studies that have addressed the effect of diabetes on COVID-19 severity independently of these comorbidities have not provided univocal results. Petrilli et al. showed that diabetes and the associated comorbidities were all independent predictors of hospital admission [29]. Likewise, Yan et al. showed that diabetes remained as independent predictor of death after adjustment for confounders including hypertension and CVD (HR, 1.53 [95% CI, 1.02–2.30] p = 0.041) in critically ill patients [62] and Guan et al. reported that, after adjusting for age and smoking status, patients with diabetes (HR, 1.59 [95% CI, 1.03–2.45]), hypertension (1.58 [1.07–2.32]), COPD (2.68 [1.42–5.05]), and malignancy (3.50 [1.60–7.64]) were more likely to reach the composite endpoint (ICU admission, invasive ventilation, or death) than those without [15]. Conversely, Zhou et al. showed that diabetes, hypertension, CHD, and CKD were significantly associated with death in univariable, but not multivariable analysis in critically ill patients [65]. Likewise, Cummings et al. showed that diabetes, hypertension, CVD, CKD, and obesity were associated with mortality in univariable analysis, but only hypertension (HR 1.58, [95% CI, 0.89–2.81]) and CVD (1.76 [1.08–2.86]) remained as independent predictor of deaths in multivariable analysis in COVID-19 patients [31]. Moreover, Wang et al. showed that hypertension, CVD, and CKD, but not diabetes, were significantly associated with mortality in univariable analysis, whereas only CVD remained as independent predictor of death in multivariable analysis in elderly patients with COVID-19 [66]. Similar results were reported in COVID-19 patients admitted to a Milan hospital, showing that hypertension, CHD, and CKD, but not diabetes, were associated with mortality in univariable analysis, whereas only CHD remained as independent predictor of death in multivariable analysis [27]. Among 2215 COVID-19 patients admitted to ICUs at 65 US hospitals, diabetes (OR, 1.14 [95% CI, 0.91–1.43]) and hypertension (1.06 [0.83–1.36]) were not independent predictors of death at multivariable analysis, at variance with age, male gender, morbid obesity, CHD, renal impairment, and active cancer [67]. Conversely, an Italian retrospective, observational study on 3988 individuals admitted to ICUs in the Lombardy region showed that diabetes remained associated with death at multivariable analysis (HR, 1.18 [95% CI, 1.01–1.39], p = 0.04), together with age, male gender, hypercholesterolemia, and COPD, but at variance with hypertension, CVD, and malignancy [68]. Finally, in the CORONADO study, body mass index (BMI) was the sole independent predictor of the primary composite outcome (OR, 1.28 [95% CI, 1.10, 1.47]), at variance with glycemic control, in patients with type 2 diabetes [40], whereas in a UK population study, the adjusted risk of death increased significantly not only for higher HbA1c, but also for an elevated BMI and an estimated glomerular filtration rate < 60 ml/min/1.73 m2 in patients with both type 1 and type 2 diabetes [38].
Overall, these observations point to a major role of the common milieu that characterizes diabetes and the associated comorbidities/complications in conferring an increased susceptibility to develop a more severe disease, with a possible additional impact of poor glycemic control. In fact, individuals with diabetes, especially those with type 2, usually present with the metabolic syndrome, i.e., with associated hypertension and/or obesity and a higher prevalence of CVD and CKD, as compared with those without diabetes. All these conditions are characterized by RAS activation and systemic inflammation and hypercoagulability that contribute to COVID-19 progression to severe and eventually fatal disease. In this context, insulin resistance, the common denominator of the metabolic syndrome, may play a major role as a pro-inflammatory and pro-coagulant trigger [69]. This concept is consistent with the observation that an index of insulin resistance, the triglyceride glucose index, was found to be associated with disease severity and mortality [70]. In addition, individuals suffering from these comorbidities are more susceptible to the occurrence of several conditions complicating the course of COVID-19, such as myocardial injury, coronary events, arrhythmias, and heart failure as well as acute kidney injury. These acute complications of COVID-19 further aggravate the underlying cardiac and renal pathologies and may eventually end-up with multi-organ failure and death. In addition, CVD and CKD may cause congestion of the pulmonary circulation, thus aggravating respiratory insufficiency, in addition to worsening tissue hypoxia and impairing compensation for acid–base imbalances.
Conclusions
The available data from the literature indicate that diabetes is not a risk factor for COVID-19, though it exerts a detrimental effect on its course and outcome, being more prevalent in patients with critical or fatal illness than in those without. However, further studies are required to clarify the mechanisms underlying the increased disease severity in diabetic versus non-diabetic individuals. In particular, it is still unresolved whether is diabetes per se, especially if poorly controlled, or rather the various comorbidities/complications associated with it that predispose patients with COVID-19 to a worse prognosis.
References
Zhu N, Zhang D, Wang W et al (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. https://doi.org/10.1056/NEJMoa2001017
Drosten C, Günther S, Preiser W et al (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348:1967–1976. https://doi.org/10.1056/NEJMoa030747
Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA (2012) Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367:1814–1820. https://doi.org/10.1056/NEJMoa1211721
World Health Organization (2020) WHO coronavirus disease (COVID-19) Dashboard. https://covid19.who.int/. Accessed 26 June 2020
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC (2020) Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. https://doi.org/10.1001/jama.2020.12839
Chen G, Wu D, Guo W et al (2020) Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Investig 130:2620–2629. https://doi.org/10.1172/JCI137244
Wang F, Nie J, Wang H et al (2020) Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis 221:1762–1769. https://doi.org/10.1093/infdis/jiaa150
Mehta P, McAuley DF, Brown M et al (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395:1033–1034. https://doi.org/10.1016/S0140-6736(20)30628-0
Tang N, Li D, Wang X, Sun Z (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18:844–847. https://doi.org/10.1111/jth.14768
Ackermann M, Verleden SE, Kuehnel M et al (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 383:120–128. https://doi.org/10.1056/NEJMoa2015432
Gudbjartsson DF, Helgason A, Jonsson H et al (2020) Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med 382:2302–2315. https://doi.org/10.1056/NEJMoa2006100
Jain V, Yuan JM (2020) Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health 25:1–14. https://doi.org/10.1007/s00038-020-01390-7
Tian W, Jiang W, Yao J et al (2020) Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. https://doi.org/10.1002/jmv.26050
Guan WJ, Ni ZY, Hu Y et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720. https://doi.org/10.1056/NEJMoa2002032
Guan WJ, Liang WH, Zhao Y et al (2020) Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 55:2000547. https://doi.org/10.1183/13993003.00547-2020
Zhu L, She ZG, Cheng X et al (2020) Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 31:1068–1077.e3. https://doi.org/10.1016/j.cmet.2020.04.021
Novel Coronavirus Pneumonia Emergency Response Epidemiology Team (2020) Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China. China CDC Weekly. https://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51. Accessed 26 June 2020
Wang B, Li R, Lu Z, Huang Y (2020) Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 12:6049–6057. https://doi.org/10.18632/aging.103000
Yang J, Zheng Y, Gou X et al (2020) Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 94:91–95. https://doi.org/10.1016/j.ijid.2020.03.017
Li B, Yang J, Zhao F et al (2020) Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 109:531–538. https://doi.org/10.1007/s00392-020-01626-9
Fadini GP, Morieri ML, Longato E, Avogaro A (2020) Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Investig 43:867–869. https://doi.org/10.1007/s40618-020-01236-2
Zheng Z, Peng F, Xu B et al (2020) Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. https://doi.org/10.1016/j.**f.2020.04.021
Hu Y, Sun J, Dai Z et al (2020) Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol 127:104371. https://doi.org/10.1016/j.jcv.2020.104371
Wang L, Gao P, Zhang M et al (2017) Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 317:2515–2523. https://doi.org/10.1001/jama.2017.7596
Kumar A, Arora A, Sharma P et al (2020) Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr 14:535–545. https://doi.org/10.1016/j.dsx.2020.04.044
Longato E, Di Camillo B, Sparacino G, Saccavini C, Avogaro A, Fadini GP (2020) Diabetes diagnosis from administrative claims and estimation of the true prevalence of diabetes among 4.2 million individuals of the Veneto region (North East Italy). Nutr Metab Cardiovasc Dis 30:84–91. https://doi.org/10.1016/j.numecd.2019.08.017
Ciceri F, Castagna A, Rovere-Querini P et al (2020) Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol 217:108509. https://doi.org/10.1016/j.clim.2020.108509
de Abajo FJ, Rodríguez-Martín S, Lerma V et al (2020) Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet 395:1705–1714. https://doi.org/10.1016/S0140-6736(20)31030-8
Petrilli CM, Jones SA, Yang J et al (2020) Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 369:m1966. https://doi.org/10.1136/bmj.m1966
Richardson S, Hirsch JS, Narasimhan M et al (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized witH COVID-19 in the New York City area. JAMA 323:2052–2059. https://doi.org/10.1001/jama.2020.6775
Cummings MJ, Baldwin MR, Abrams D et al (2020) Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 395:1763–1770. https://doi.org/10.1016/S0140-6736(20)31189-2
Argenziano MG, Bruce SL, Slater CL et al (2020) Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ 369:m1996. https://doi.org/10.1136/bmj.m1996
Myers LC, Parodi SM, Escobar GJ, Liu VX (2020) Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA 323:2195–2198. https://doi.org/10.1001/jama.2020.7202
Garg S, Kim L, Whitaker M et al (2020) Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep 69:458–464. https://doi.org/10.15585/mmwr.mm6915e3
Centers for Disease Control and Prevention (2020) National diabetes statistics report, 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 14 July 2020
CDC COVID-19 Response Team (2020) Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep 69:382–386. https://doi.org/10.15585/mmwr.mm6913e2
SARS-CoV-2 Surveillance Group (2020) Characteristics of SARS-CoV-2 patients dying in Italy. Report based on available data on July 9th, 2020. https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_9_july_2020.pdf. Accessed 26 June 2020
Holman N, Knighton P, Kar P et al (2020) Type 1 and Type 2 diabetes and COVID-19 related mortality in England: a cohort study in people with diabetes. Pre-print. https://www.england.nhs.uk/wp-content/uploads/2020/05/Valabhji-COVID-19-and-Diabetes-Paper-2-Full-Manuscript.pdf. Accessed 26 June 2020
Huang I, Lim MA, Pranata R (2020) Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr 14:395–403. https://doi.org/10.1016/j.dsx.2020.04.018
Cariou B, Hadjadj S, Wargny M et al (2020) Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia 63:1500–1515. https://doi.org/10.1007/s00125-020-05180-x
Rubino F, Amiel SA, Zimmet P et al (2020) New-onset diabetes in Covid-19. N Engl J Med. https://doi.org/10.1056/NEJMc2018688
Liu Q, Chen H, Li J et al (2020) Fasting blood glucose predicts the occurrence of critical illness in COVID-19 patients: a multicenter retrospective cohort study. J Infect. https://doi.org/10.1016/j.**f.2020.07.006
Wang S, Ma P, Zhang S et al (2020) Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia. https://doi.org/10.1007/s00125-020-05209-1
Zhang B, Liu S, Zhang L, Dong Y, Zhang S (2020) Admission fasting blood glucose predicts 30-day poor outcome in patients hospitalized for COVID-19 pneumonia. Diabetes Obes Metab. https://doi.org/10.1111/dom.14132
Bornstein SR, Rubino F, Khunti K et al (2020) Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol 8:546–550. https://doi.org/10.1016/S2213-8587(20)30152-2
Fadini GP, Morieri ML, Longato E et al (2020) Exposure to dipeptidyl-peptidase-4 inhibitors and COVID-19 among people with type 2 diabetes: a case-control study. Diabetes Obes Metab. https://doi.org/10.1111/dom.14097
Drucker DJ (2020) Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev 41(3):bnaa011. https://doi.org/10.1210/endrev/bnaa011
Song W, Wang Y, Wang N et al (2014) Identification of residues on human receptor DPP4 critical for MERS-CoV binding and entry. Virology 471–473:49–53. https://doi.org/10.1016/j.virol.2014.10.006
Letko M, Marzi A, Munster V (2020) Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol 5:562–569. https://doi.org/10.1038/s41564-020-0688-y
Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G (2020) Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med 382:2431–2440. https://doi.org/10.1056/NEJMoa2006923
Reynolds HR, Adhikari S, Pulgarin C et al (2020) Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med 382:2441–2448. https://doi.org/10.1056/NEJMoa2008975
Fosbøl EL, Butt JH, Østergaard L et al (2020) Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA 324:168–177
Bavishi C, Maddox TM, Messerli FH (2020) Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA. https://doi.org/10.1001/jama.2020.11301
Imai Y, Kuba K, Rao S et al (2005) Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436:112–116. https://doi.org/10.1038/nature03712
Zhang P, Zhu L, Cai J et al (2020) Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 126:1671–1681. https://doi.org/10.1161/CIRCRESAHA.120.317134
Gao C, Cai Y, Zhang K et al (2020) Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J 41:2058–2066. https://doi.org/10.1093/eurheartj/ehaa433
Pirola CJ, Sookoian S (2020) Estimation of renin-angiotensin-aldosterone-system (RAAS)-inhibitor effect on COVID-19 outcome: a meta-analysis. J Infect. https://doi.org/10.1016/j.**f.2020.05.052
Ji HL, Zhao R, Matalon S, Matthay MA (2020) Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev 100:1065–1075. https://doi.org/10.1152/physrev.00013.2020
Gupta R, Ghosh A, Singh AK, Misra A (2020) Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab Syndr 14:211–212. https://doi.org/10.1016/j.dsx.2020.03.002
Hotamisligil GS (2017) Inflammation, metaflammation and immunometabolic disorders. Nature 542:177–185. https://doi.org/10.1038/nature21363
Muniyappa R, Gubbi S (2020) COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol 318:E736–E741. https://doi.org/10.1152/ajpendo.00124.2020
Yan Y, Yang Y, Wang F et al (2020) Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care 8:e001343. https://doi.org/10.1136/bmjdrc-2020-001343
Guo W, Li M, Dong Y et al (2020) Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev 31:e3319. https://doi.org/10.1002/dmrr.3319
Wang Z, Du Z, Zhu F (2020) Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID-19 patients. Diabetes Res Clin Pract 164:108214. https://doi.org/10.1016/j.diabres.2020.108214
Zhou F, Yu T, Du R et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3
Wang L, He W, Yu X et al (2020) Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect 80:639–645. https://doi.org/10.1016/j.**f.2020.03.019
Gupta S, Hayek SS, Wang W et al (2020) Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. https://doi.org/10.1001/jamainternmed.2020.3596
Grasselli G, Greco M, Zanella A et al (2020) Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. https://doi.org/10.1001/jamainternmed.2020.3539
Sakkinen PA, Wahl P, Cushman M, Lewis MR, Tracy RP (2000) Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome. Am J Epidemiol 152:897–907. https://doi.org/10.1093/aje/152.10.897
Ren H, Yang Y, Wang F et al (2000) Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc Diabetol 19:58. https://doi.org/10.1186/s12933-020-01035-2
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization was contributed by GP and EO. Data curation (literature search and analysis) was contributed by GP, MV, VR, and EO. Writing—original draft, was contributed by GP. Writing—review and editing, was contributed by MV, VR, and EO.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
Not applicable.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pugliese, G., Vitale, M., Resi, V. et al. Is diabetes mellitus a risk factor for COronaVIrus Disease 19 (COVID-19)?. Acta Diabetol 57, 1275–1285 (2020). https://doi.org/10.1007/s00592-020-01586-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-020-01586-6