Summary
Objective
To investigate the genetic level causal association among hyperthyroidism, hypothyroidism, and rheumatoid arthritis (RA).
Methods
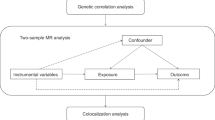
We utilized the genome-wide association studies (GWAS) summary data for exposure (hyperthyroidism and hypothyroidism) and outcome (RA) from the IEU OpenGWAS database. We used two different sets of data (test cohort and validation cohort) for causal assessment of exposure and outcome. To establish a causal relationship between these conditions, we conducted a two-sample Mendelian randomization (MR) analysis. Subsequently, we evaluated the MR analysis results for heterogeneity, horizontal pleiotropy, and outliers, aiming to assess the validity and reliability of the findings. Moreover, we conducted additional analyses to examine the robustness of the MR results, including a “Leave one out” analysis and the MR robust adjusted profile score (MR-RAPS) method, ensuring the robustness and adherence to normal distribution assumptions.
Results
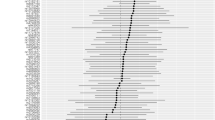
The findings from the test cohort indicated that hyperthyroidism did not exhibit a genetic causal association with RA (P = 0.702, odds ratio [OR] 95% confidence interval [CI] = 1.021 [0.918–1.135]). Conversely, hypothyroidism displayed a positive genetic causal relationship with RA (P < 0.001, OR 95% CI = 1.239 [1.140–1.347]). The analysis results of the validation cohort are consistent with those of the test cohort. Notably, our MR analysis results demonstrated no evidence of heterogeneity, horizontal pleiotropy, or outliers. Furthermore, our MR analysis results remained unaffected by any single nucleotide polymorphism (SNP) and exhibited a normal distribution.
Conclusion
The results of this study showed that hypothyroidism was positively correlated with RA, while hyperthyroidism was not causally correlated with RA. Hypothyroidism may as a risk factor of RA should be paid attention to in clinical work. Future studies are needed to further confirm this finding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by symmetrical multiarticular synovitis. Inflammatory cell infiltration results in the degeneration of synovitis, articular cartilage, and bone [1, 2]. RA primarily exhibits chronic, symmetrical, multisynovial arthritis and extra-articular lesions, predominantly affecting small joints such as the hands, wrists, and feet, with recurrent and symmetrical symptoms [3]. RA patients may experience manifestations in other organs, including interstitial lung disease, pericarditis, pleural effusion, or bronchiectasis [3]. The incidence of RA ranges from 0.5 to 1%, with a male-to-female ratio of 2.5:1. Although RA can occur at any age, it most commonly emerges between 40 and 70 years old. The age of onset is positively associated with the incidence of RA, and approximately 40% of RA patients become disabled within 10 years [4]. Presently, genetic and environmental factors are recognized as the primary regulatory factors for RA [5]. Individuals with a positive family history have a 3- to 5‑fold increased risk of develo** RA [6]. The heritability of serological positive RA is estimated to be 40–65%, whereas serological negative RA has a heritability of 20% [7, 8]. Currently, there is no cure for RA. In advanced stages of the disease, surgical interventions, such as total knee replacement and total hip replacement, are necessary for large joint management. The chronic joint inflammation associated with RA leads to joint damage, functional loss, and various musculoskeletal impairments, ultimately resulting in decreased physical and social functioning in patients. Moreover, advanced RA not only causes joint destruction and disability but also contributes to multiple secondary complications, including cardiovascular disease, significantly impacting both the quality of life for patients and society as a whole.
Hyperthyroidism is a clinical syndrome characterized by an excessive production and secretion of thyroid hormones by the thyroid gland, resulting in a state of hypermetabolism. This condition is distinguished by elevated levels of free serum thyroxine (T4) and/or free triiodothyronine (T3) [9]. Hyperthyroidism is a prevalent disorder worldwide, with a global prevalence ranging from 0.2–1.3% [10]. In the United States, the overall prevalence of hyperthyroidism is reported as 1.2%, with dominant hyperthyroidism accounting for 0.5% and subclinical hyperthyroidism accounting for 0.7% [11]. The primary causes of hyperthyroidism are commonly attributed to Graves’ disease (GD), toxic multinodular goiters, and toxic adenomas [11]. Clinical manifestations of hyperthyroidism encompass a range of symptoms, including increased heat production and basal metabolic rate due to cellular effects mediated by T3-binding receptors, resulting in weight loss and fatigue. Additionally, individuals with GD may exhibit skin changes such as thinning hair and anterior tibial mucous edema [11]. Hyperthyroidism can significantly impact various systems in the body, including the integumentary, musculoskeletal, immune, ophthalmic, reproductive, gastrointestinal, and cardiovascular systems, posing a substantial threat to overall human health [11]. Hypothyroidism, on the other hand, is a frequently encountered clinical disorder and the most prevalent hormonal deficiency disorder. It occurs when the thyroid gland fails to produce adequate amounts of thyroid hormone to meet the demands of surrounding tissues [12]. In its early stages, hypothyroidism often remains latent and may exhibit no noticeable symptoms. However, as the disease progresses, nonspecific symptoms begin to manifest, commonly including fatigue, cold tolerance, and constipation [13]. Thus, the clinical presentation of hypothyroidism can vary significantly [14]. Hypothyroidism ranges in severity from asymptomatic cases to severe conditions leading to coma and multiple organ failure [15]. It is more commonly observed in women, with the incidence increasing with age [15]. Overt hypothyroidism has a prevalence ranging from 0.3–3.7% in the United States and from 0.2–5.3% in Europe. Subclinical hypothyroidism exhibits an even higher prevalence, estimated to be between 3–15% [12]. If left untreated, severe hypothyroidism can result in heart failure, psychosis, and coma, thus imposing considerable distress on individuals and society at large [15].
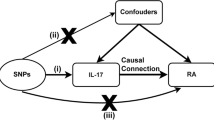
Genome-wide association studies (GWAS) have significantly advanced the field of human disease genetics, and the publicly available GWAS summary data provides a vast database for further genetic investigations into various diseases and traits [16]. Mendelian randomization (MR) is a statistical approach employed for inferring epidemiological etiology analysis, following Mendel’s law of heredity, which entails the random assignment of alleles. Utilizing extensive GWAS summary data, MR analysis utilizes single nucleotide polymorphisms (SNPs) as instrumental variables (IVs) to investigate genetic causality between exposure and outcome [17]. MR effectively controls for confounding factors by leveraging the innate nature of genetic mutations, which remain unaffected by environmental influences. Furthermore, as genetic variations can influence outcomes, but outcomes cannot impact genes, MR avoid the issue of reverse causation [18]. Previous studies have identified a causal relationship between hyperthyroidism and venous thromboembolism through MR analysis [29, 30]. Conversely, other reports have indicated a higher prevalence of AITDs in patients with RA [28, 31], or an increased occurrence of thyroid autoantibodies in individuals with RA [32].
RA primarily affects the joints, but it can also involve other systems such as the cardiovascular system, skin, uvea, lungs, and other extra-articular organs. While the exact cause of RA remains incompletely understood, current understanding recognizes the intricate interplay between genetic susceptibility and environmental factors. It is believed that the combined influence of multiple factors contributes to the onset and progression of RA [33]. RA is fundamentally an autoimmune disease, and it is plausible that the etiology of AITD, another autoimmune condition, shares similarities with RA. Although the precise mechanism underlying the shared origins of AITD and RA remains uncertain, both genetic and environmental factors are generally acknowledged as significant contributors to the association between these conditions [34]. Previous investigations have also examined the prevalence of thyroid disease among RA patients in different geographical locations. The prevalence of thyroid disease among RA patients ranged from 0.5% in Morocco to 27% in Slovakia [32, 35]. Similarly, the prevalence of thyroid autoantibodies in RA patients is reported as 5% in the United Kingdom and nearly 30% in Japan [36, 37]. These findings provide compelling evidence for the involvement of genetic susceptibility and environmental factors in the development of autoimmune diseases across diverse populations, supporting the association between AITD and RA.
Both hyperthyroidism and hypothyroidism have been extensively linked to RA. Numerous previous observational studies have consistently reported a stronger correlation between hypothyroidism and RA compared to hyperthyroidism, indicating that hypothyroidism is a more prevalent thyroid dysfunction among RA patients [33]. In this study, we employed MR analysis to investigate the genetic causal relationship between hyperthyroidism, hypothyroidism, and RA at the genetic level. Our findings further confirmed a causal relationship between hypothyroidism and RA, exhibiting a positive genetic causal association. Conversely, no genetic causal relationship was observed between hyperthyroidism and RA. These results align, to some extent, with previous research findings. Notably, hypothyroidism exhibits a stronger association with RA than hyperthyroidism in terms of thyroid dysfunction. We postulate that this disparity may be attributed to the relatively lower immunogenicity of hyperthyroidism compared to hypothyroidism [33]. The majority of hypothyroidism cases stem from autoimmune-mediated Hashimoto’s disease, whereas the prevalence of autoimmune diseases leading to hyperthyroidism ranges from 50 to 80%, depending on factors such as age, geographical region, and iodine intake [38].
There are several limitations to be acknowledged in this study. Firstly, it is important to note that the study population consisted exclusively of individuals from European descent. Given the potential impact of genetic susceptibility and environmental factors, caution should be exercised when generalizing the findings of this study to other populations. Secondly, hyperthyroidism, hypothyroidism, and RA exhibit gender disparities. Although our study did not differentiate between genders due to limitations in the available data, future investigations should consider exploring potential differences in the results when applied specifically to male or female populations.
Conclusion
This study aimed to investigate the genetic-level causal relationship between hyperthyroidism, hypothyroidism, and RA. The findings revealed that hypothyroidism exhibited a positive causal association with RA, whereas hyperthyroidism did not demonstrate any causal relationship with RA. However, additional investigations are warranted to elucidate their precise connections across different levels in subsequent studies.
Availability of data and materials
Publicly available datasets were analyzed in this study. These data can be found here: IEU OpenGWAS database (https://gwas.mrcieu.ac.uk/).
References
Sun HT, Li JP, Qian WQ, Yin MF, Yin H, Huang GC. Quercetin suppresses inflammatory cytokine production in rheumatoid arthritis fibroblast-like synoviocytes. Exp Ther Med. 2021;22(5):1260.
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38.
Sparks JA. Rheumatoid Arthritis. Ann Intern Med. 2019;170(1):ITC1–ITC16.
Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903–11.
Holoshitz J. The rheumatoid arthritis HLA-DRB1 shared epitope. Curr Opin Rheumatol. 2010;22(3):293–8.
Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002;4(Suppl 3):265–72.
Jiang X, Frisell T, Askling J, Karlson EW, Klareskog L, Alfredsson L, et al. To what extent is the familial risk of rheumatoid arthritis explained by established rheumatoid arthritis risk factors? Arthritis & rheumatology (Hoboken, NJ). 2015;67(2):352–62.
Frisell T, Hellgren K, Alfredsson L, Raychaudhuri S, Klareskog L, Askling J. Familial aggregation of arthritis-related diseases in seropositive and seronegative rheumatoid arthritis: a register-based case-control study in Sweden. Ann Rheum Dis. 2016;75(1):183–9.
LiVolsi VA, Baloch ZW. The Pathology of Hyperthyroidism. Front Endocrinol (lausanne). 2018;9:737.
Wiersinga WM, Poppe KG, Hyperthyroidism EG. aetiology, pathogenesis, diagnosis, management, complications, and prognosis. Lancet Diabetes Endocrinol. 2023;11(4):282–98.
Doubleday AR, Hyperthyroidism SRS. Gland Surg. 2020;9(1):124–35.
McDermott MT. Hypothyroidism. Ann Intern Med. 2020;173(1):ITC1–ITC16.
Chaker L, Razvi S, Bensenor IM, Azizi F, Pearce EN, Hypothyroidism PRP. Nat Rev Dis Primers. 2022;8(1):30.
Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550–62.
Roberts CG, Ladenson PW. Hypothyroidism. Lancet. 2004;363(9411):793–803.
Wang Y, Guo P, Zhang Y, Liu L, Yan R, Yuan Z, et al. Joint Analysis of Genetic Correlation, Mendelian Randomization and Colocalization Highlights the Bi-Directional Causal Association Between Hypothyroidism and Primary Biliary Cirrhosis. Front Genet. 2021;12:753352.
Qin Q, Zhao L, Ren A, Li W, Ma R, Peng Q, et al. Systemic lupus erythematosus is causally associated with hypothyroidism, but not hyperthyroidism: A Mendelian randomization study. Front Immunol. 2023;14:1125415.
Lu L, Wan B, Li L, Sun M. Hypothyroidism has a protective causal association with hepatocellular carcinoma: A two-sample Mendelian randomization study. Front Endocrinol (lausanne). 2022;13:987401.
Han F, Zhang C, Xuan M, **e Z, Zhang K, Li Y. Effects of Hyperthyroidism on Venous Thromboembolism: A Mendelian Randomization Study. J Immunol Res. 2022;2022:2339678.
Cai Y, Zhang G, Liang J, **g Z, Zhang R, Lv L, et al. Causal Relationships Between Osteoarthritis and Senile Central Nerve System Dysfunction: A Bidirectional Two-Sample Mendelian Randomization Study. Front Aging Neurosci. 2021;13:793023.
Meng H, Jiang L, Song Z, Wang F. Causal Associations of Circulating Lipids with Osteoarthritis: A Bidirectional Mendelian Randomization Study. Nutrients. 2022;14(7):1327.
Zhang Y, Fan J, Chen L, **ong Y, Wu T, Shen S, et al. Causal Association of Coffee Consumption and Total, Knee, Hip and Self-Reported Osteoarthritis: A Mendelian Randomization Study. Front Endocrinol (lausanne). 2021;12:768529.
Li C, Niu M, Guo Z, Liu P, Zheng Y, Liu D, et al. A Mild Causal Relationship Between Tea Consumption and Obesity in General Population: A Two-Sample Mendelian Randomization Study. Front Genet. 2022;13:795049.
Cao Z, Wu Y, Li Q, Li Y, Wu J. A causal relationship between childhood obesity and risk of osteoarthritis: results from a two-sample Mendelian randomization analysis. Ann Med. 2022;54(1):1636–45.
Waldenlind K, Saevarsdottir S, Bengtsson C, Askling J. Risk of Thyroxine-Treated Autoimmune Thyroid Disease Associated With Disease Onset in Patients With Rheumatoid Arthritis. Jama Netw Open. 2018;1(6):e183567.
Becker KL, Ferguson RH, McConahey WM. The connective-tissue diseases and symptoms associated with Hashimoto’s thyroiditis. N Engl J Med. 1963;268:277–80.
Boelaert K, Newby PR, Simmonds MJ, Holder RL, Carr-Smith JD, Heward JM, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123(2):183:e1–9.
Biro E, Szekanecz Z, Czirjak L, Danko K, Kiss E, Szabo NA, et al. Association of systemic and thyroid autoimmune diseases. Clin Rheumatol. 2006;25(2):240–5.
Bengtsson C, Padyukov L, Kallberg H, Saevarsdottir S. Thyroxin substitution and the risk of develo** rheumatoid arthritis; results from the Swedish population-based EIRA study. Ann Rheum Dis. 2014;73(6):1096–100.
Kerola AM, Nieminen TVM, Kauppi MJ, Kautiainen H, Puolakka K, Virta LJ, et al. Increased risk of levothyroxine-treated hypothyroidism preceding the diagnosis of rheumatoid arthritis: a nationwide registry study. Clin Exp Rheumatol. 2014;32(4):455–9.
Cardenas Roldan J, Amaya-Amaya J, Castellanos-de la Hoz J, Giraldo-Villamil J, Montoya-Ortiz G, Cruz-Tapias P, et al. Autoimmune thyroid disease in rheumatoid arthritis: a global perspective. Arthritis. 2012;2012:864907.
Lazurova I, Benhatchi K, Rovensky J, Kozakova D, Wagnerova H, Tajtakova M, et al. Autoimmune thyroid disease and autoimmune rheumatic disorders: a two-sided analysis. Ann N Y Acad Sci. 2009;1173:211–6.
Mahagna H, Caplan A, Watad A, Bragazzi NL, Sharif K, Tiosano S, et al. Rheumatoid arthritis and thyroid dysfunction: A cross-sectional study and a review of the literature. Best Pract Res Clin Rheumatol. 2018;32(5):683–91.
Nazary K, Hussain N, Ojo RO, Anwar S, Kadurei F, Hafizyar F, et al. Prevalence of Thyroid Dysfunction in Newly Diagnosed Rheumatoid Arthritis Patients. Cureus. 2021;13(9):e18204.
Benamour S, Zeroual B, Fares L, Kabli H, Bettal S. Rheumatoid arthritis in Morocco. Apropos of 404 observations. Rev Rhum Mal Osteoartic. 1992;59(12):801–7.
Silman AJ, Ollier WE, Bubel MA. Autoimmune thyroid disease and thyroid autoantibodies in rheumatoid arthritis patients and their families. Br J Rheumatol. 1989;28(1):18–21.
Nakamura H, Usa T, Motomura M, Ichikawa T, Nakao K, Kawasaki E, et al. Prevalence of interrelated autoantibodies in thyroid diseases and autoimmune disorders. J Endocrinol Invest. 2008;31(10):861–5.
Cooper DS. Hyperthyroidism. Lancet. 2003;362(9382):459–68.
Author information
Authors and Affiliations
Contributions
Peng Xu and Mingyi Yang designed the study. Mingyi Yang, Yani Su and Ke Xu analyzed the datasets and interpreted the results. Mingyi Yang, Pengfei Wen and Jianbin Guo downloaded the data. Zhi Yang and Lin Liu provided software support. Mingyi Yang wrote and edited the manuscript. Peng Xu provided the support.
Corresponding author
Ethics declarations
Conflict of interest
M. Yang, Y. Su, K. Xu, P. Wen, J. Guo, Z. Yang, L. Liu and P. Xu declare that they have no competing interests.
Ethical standards
Consent for publication: All authors read and approved the final manuscript. And agree to the publication of the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
508_2024_2386_MOESM1_ESM.docx
Supplementary information provides details of the data used in this study. And details of IVs for which exposure and outcome were evaluated for genetic causality, including SNPs excluded during the IVs screening process. These include SNPs that are associated with outcome, confounding SNPs, and palindromic SNPs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, M., Su, Y., Xu, K. et al. A causal relationship between hypothyroidism and rheumatoid arthritis, but not hyperthyroidism: evidence from the mendelian randomization study. Wien Klin Wochenschr (2024). https://doi.org/10.1007/s00508-024-02386-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00508-024-02386-6