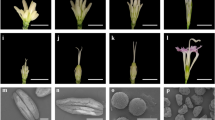
Key message
Candidate male sterility genes were identified in sugarcane, which interacts with kinase-related proteins, transcription factors, and plant hormone signaling pathways to regulate stamen and anther development.
Abstract
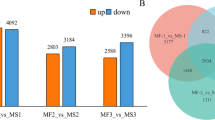
Saccharum officinarum is a cultivated sugarcane species that its predominant feature is high sucrose content in stems. Flowering is necessary for breeding new cultivars but will terminate plant growth and reduce sugar yield. The wild sugarcane species Saccharum spontaneum has robust and viable pollen, whereas most S. officinarum accessions are male sterile, which is a desirable trait of a maternal parent in sugarcane breeding. To study male sterility and related regulatory pathways in sugarcane, we carried out RNAseq using flowers in different developmental stages between male-sterile S. officinarum accession ‘LA Purple’ and fertile S. spontaneum accession ‘SES208’. Gene expression profiles were used to detect how genes are differentially expressed between male sterile and fertile flowers and to identify candidate genes for male sterility. Weighted gene correlation networks analysis (WGCNA) was conducted to investigate the regulatory networks. Transcriptomic analyses showed that 988 genes and 2888 alleles were differentially expressed in S. officinarum compared to S. spontaneum. Ten differentially expressed genes and thirty alleles were identified as candidate genes and alleles for male sterility in sugarcane. The gene Sspon.03G0007630 and two alleles of the gene Sspon.08G0002270, Sspon.08G0002270-2B and Sspon.08G0014700-1A, were involved in the early stamen or carpel development stages, while the remaining genes were classified into the post-meiosis stage. Gibberellin, auxin, and jasmonic acid signaling pathways are involved in the stamen development in sugarcane. The results expanded our knowledge of male sterility-related genes in sugarcane and generated genomic resources to facilitate the selection of ideal maternal parents to improve breeding efficiency.









Similar content being viewed by others
Data availability
The RNAseq data generated during the current study are available in the Sequence Read Archive (SRA, https://www.ncbi.nlm.nih.gov/sra/) under the BioProject accession number: PRJNA1051631.
References
Acosta IF, Laparra H, Romero SP, Schmelz E, Hamberg M, Mottinger JP, Dellaporta SL (2009) tasselseed1 is a lipoxygenase affecting jasmonic acid signaling in sex determination of maize. Science 323(5911):262–265
Bensen RJ, Johal GS, Crane VC, Tossberg JT, Schnable PS, Meeley RB, Briggs SP (1995) Cloning and characterization of the maize An1 gene. Plant Cell 7(1):75–84
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120
Bremer G (1923) A cytological investigation of some species and species hybrids within the genus Saccharum. Genetica 5:273–326
Bremer G (1961) Problems in breeding and cytology of sugarcane. III. The cytological crossing research of sugarcane. Euphytica 10:229–243
Bull TA, Glasziou KT (1975) Sugarcane. In: Evans LT (ed) Crop physiology: some case histories. Cambridge University Press, London, pp 51–72
Cheavegatti-Gianotto A, De Abreu HMC, Arruda P, Bespalhok Filho JC, Burnquist WL, Creste S, César Ulian E (2011) Sugarcane (Saccharum X officinarum): a reference study for the regulation of genetically modified cultivars in Brazil. Trop Plant Biol 4:62–89
Chen L, Liu YG (2014) Male sterility and fertility restoration in crops. Annu Rev Plant Biol 65:579–606
Clements HF (1975) Flowering of sugarcane: mechanics and control
D’Hont A, Lu YH, Feldmann P, Glaszmann JC (1993) Cytoplasmic diversity in sugar cane revealed by heterogous probes
Denyer T, Ma X, Klesen S, Scacchi E, Nieselt K, Timmermans MC (2019) Spatiotemporal developmental trajectories in the Arabidopsis root revealed using high-throughput single-cell RNA sequencing. Dev Cell 48(6):840–852
Diggle PK, Di Stilio VS, Gschwend AR, Golenberg EM, Moore RC, Russell JR, Sinclair JP (2011) Multiple developmental processes underlie sex differentiation in angiosperms. Trends Genet 27(9):368–376
Divinagracia NS (1982) Emasculation of sugarcane flowers: steam method. Techn Inf Digest (Philippines): 26
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29(1):15–21
Domagalska MA, Schomburg FM, Amasino RM, Vierstra RD, Nagy F, Davis SJ (2007) Attenuation of brassinosteroid signaling enhances FLC expression and delays flowering
Fechter I, Hausmann L, Daum M, Rosleff Sörensen T, Viehöver P, Weisshaar B, Töpfer R (2012) Candidate genes within a 143 kb region of the flower sex locus in Vitis. Mol Genet Genomics 287:247–259
Griffiths J, Murase K, Rieu I, Zentella R, Zhang ZL, Powers SJ, Thomas SG (2006) Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18(12):3399–3414
Hartwig T, Chuck GS, Fujioka S, Klempien A, Weizbauer R, Potluri DPV, Schulz B (2011) Brassinosteroid control of sex determination in maize. Proc Natl Acad Sci 108(49):19814–19819
Huerta-Cepas J, Forslund K, Coelho LP, Szklarczyk D, Jensen LJ, Von Mering C, Bork P (2017) Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Mol Biol Evol 34(8):2115–2122
Liao Y, Smyth GK, Shi W (2014) featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30(7):923–930
Liu X, Yin Z, Liu Y, Li Z, Yan M, Que Y, Zhou D (2020) The complete mitochondrial genome of sugarcane (Saccharum spp.) variety FN15. Mitochondrial DNA Part B 5(3):2163–2165
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12):1–21
Machado GP Jr (1987) Sugarcane improvement. In: Paranhos SB (ed) Sugarcane: cultivation and use. Campinas, Cargill Foundation, pp 165–186
McIntyre CL, Jackson PA (2001) Low level of selfing found in a sample of crosses in Australian sugarcane breeding programs. Euphytica 117(3):245–249
Melloni MLG, Scarpari MS, de Mendonça JR, Perecin D, de Andrade Landell MG, Pinto LR (2013) Comparison of two staining methods for pollen viability studies in sugarcane. Sugar Tech 15:103–107
Midmore DJ (1980) Effects of photoperiod on flowering and fertility of sugarcane (Saccharum spp.). Field Crop Res 3:65–81
Moore PH (1976) Studies on sugarcane pollen. II. Pollen Storage
Moore PH, Nuss KJ (1987) Flowering and flower synchronization. In: Developments in crop science, Elsevier, Vol. 11, pp 273–311
Moore PH, Botha FC (eds) (2013) Sugarcane: physiology, biochemistry and functional biology. John Wiley & Sons
Moore PH, Berding N (2013) Flowering. Sugarcane Physiol Biochem Funct Biol 379-410. https://doi.org/10.1002/9781118771280.ch15
Neumann M, Xu X, Smaczniak C, Schumacher J, Yan W, Blüthgen N, Muino JM (2022) A 3D gene expression atlas of the floral meristem based on spatial reconstruction of single nucleus RNA sequencing data. Nat Commun 13(1):2838
Price S (1957) Cytological studies in Saccharum and allied genera. III. Chromosome numbers in interspecific hybrids. Botan Gazette 118(3):146–159
Qi T, Huang H, Wu D, Yan J, Qi Y, Song S, **e D (2014) Arabidopsis DELLA and JAZ proteins bind the WD-repeat/bHLH/MYB complex to modulate gibberellin and jasmonate signaling synergy. Plant Cell 26(3):1118–1133
Raghavan TS (1951) Cytogenetics of sugarcane. Indian J Agric Sci 22:93–102
Ridhawati A, Mahayu WM, Garusti G, Asbani N (2022) Viability of sugarcane and Erianthus arundinaceus pollen under marcotting treatment. In: IOP conference series: earth and environmental science, IOP Publishing, Vol. 974(1), pp 012053
Roach BT (1972) Nobilization of sugarcane. Proc Int Soc Sugar Cane Technol 14:206–216
Ryu KH, Huang L, Kang HM, Schiefelbein J (2019) Single-cell RNA sequencing resolves molecular relationships among individual plant cells. Plant Physiol 179(4):1444–1456
Sharma L, Dalal M, Verma RK, Kumar SV, Yadav SK, Pushkar S, Chinnusamy V (2018) Auxin protects spikelet fertility and grain yield under drought and heat stresses in rice. Environ Exp Bot 150:9–24
Shearman JR, Sonthirod C, Naktang C, Pootakham W, Yoocha T, Sangsrakru D, Tangphatsornruang S (2016) The two chromosomes of the mitochondrial genome of a sugarcane cultivar: assembly and recombination analysis using long PacBio reads. Sci Rep 6(1):31533
Shulse CN, Cole BJ, Ciobanu D, Lin J, Yoshinaga Y, Gouran M, Dickel DE (2019) High-throughput single-cell transcriptome profiling of plant cell types. Cell Rep 27(7):2241–2247
Sleper DA, Poehlman JM (2006) Breeding field crops (No. Ed. 5). Blackwell Publishing
Stevenson GC (1965) Genetics and breeding of sugar cane. Genetics and breeding of sugar cane
Szemenyei H, Hannon M, Long JA (2008) TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319(5868):1384–1386
Tabata R, Ikezaki M, Fujibe T, Aida M, Tian CE, Ueno Y, Ishiguro S (2010) Arabidopsis auxin response factor6 and 8 regulate jasmonic acid biosynthesis and floral organ development via repression of class 1 KNOX genes. Plant Cell Physiol 51(1):164–175
Varet H, Brillet-Guéguen L, Coppée JY, Dillies MA (2016) SARTools: a DESeq2-and EdgeR-based R pipeline for comprehensive differential analysis of RNA-seq data. PLoS ONE 11(6):e0157022
Xu Z, Wang Y, Chen Y, Yin H, Wu L, Zhao Y, Gao M (2019) A model of hormonal regulation of stamen abortion during pre-meiosis of Litsea cubeba. Genes 11(1):48
Zhang B, Horvath S (2005) A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. https://doi.org/10.2202/1544-6115.1128
Zhang J, Zhang X, Tang H, Zhang Q, Hua X, Ma X, Ming R (2018) Allele-defined genome of the autopolyploid sugarcane Saccharum spontaneum L. Nat Genet 50(11):1565–1573
Zhang TQ, Xu ZG, Shang GD, Wang JW (2019) A single-cell RNA sequencing profiles the developmental landscape of Arabidopsis root. Mol Plant 12(5):648–660
Zhou D, Yin Z, Liu X, Li Z, Yan M, Que Y, Xu L (2020) The complete mitochondrial genome sequence and phylogenetic analysis of sugarcane (Saccharum spp.) cultivar ROC22. Mitochondrial DNA Part B 5(2):1915–1916
Zhou D, Liu Y, Yao J, Yin Z, Wang X, Xu L, Liu X (2022) Characterization and phylogenetic analyses of the complete mitochondrial genome of sugarcane (Saccharum spp. hybrids) line A1. Diversity 14(5):333
Acknowledgements
We appreciate the Keck Center for sequencing service and Hawaii Agriculture Research Center for maintaining plant materials.
Author information
Authors and Affiliations
Contributions
R.M. conceived this project. R.M., X.Z., and J.S. designed the experiments. T.J. and M.W. collected plant materials. J.S. performed data analysis. J.S. and R.M. drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing financial interests.
Additional information
Communicated by Ravishankar Palanivelu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, J., Zhang, X., Jones, T. et al. Identification of male sterility-related genes in Saccharum officinarum and Saccharum spontaneum. Plant Reprod (2024). https://doi.org/10.1007/s00497-024-00503-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00497-024-00503-z