Abstract
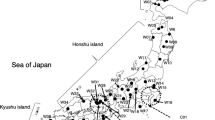
To explore the roles of plasticity and genetic variation in the response to spatial and temporal climate variation, we established a common garden consisting of paired collections of native and introduced riparian trees sampled along a latitudinal gradient. The garden in Fort Collins, Colorado (latitude 40.6°N), included 681 native plains cottonwood (Populus deltoides subsp. monilifera) and introduced saltcedar (Tamarix ramosissima, T. chinensis and hybrids) collected from 15 sites at 29.2–47.6°N in the central United States. In the common garden both species showed latitudinal variation in fall, but not spring, leaf phenology, suggesting that the latitudinal gradient in fall phenology observed in the field results at least in part from inherited variation in the critical photoperiod, while the latitudinal gradient in spring phenology observed in the field is largely a plastic response to the temperature gradient. Populations from higher latitudes exhibited earlier bud set and leaf senescence. Cold hardiness varied latitudinally in both fall and spring for both species. For cottonwood, cold hardiness began earlier and ended later in northern than in southern populations. For saltcedar northern populations were hardier throughout the cold season than southern populations. Although cottonwood was hardier than saltcedar in midwinter, the reverse was true in late fall and early spring. The latitudinal variation in fall phenology and cold hardiness of saltcedar appears to have developed as a result of multiple introductions of genetically distinct populations, hybridization and natural selection in the 150 years since introduction.



Similar content being viewed by others
References
Arora R, Rowland LJ, Tanino K (2003) Induction and release of bud dormancy in woody perennials: a science comes of age. Hortscience 38:911–921
Baum BR (1978) The genus Tamarix. Israel Academy of Sciences and Humanities, Jerusalem
Borchert R, Robertson K, Schwartz MD, Williams-Linera G (2005) Phenology of temperate trees in tropical climates. Int J Biometeorol 50:57–65
Brissette JC, Barnes BV (1984) Comparisons of phenology and growth of Michigan and western North American sources of Populus tremuloides. Can J For Res 14:789–793
Calkins JB, Swanson BT (1990) The distinction between living and dead plant tissue—viability tests in cold hardiness research. Cryobiology 27:194–211
Cannell MGR, Sheppard LJ (1982) Seasonal changes in the frost hardiness of provenances of Picea sitchensis in Scotland. Forestry 55:137–153
Chen THH, Howe GT, Bradshaw HD Jr (2002) Molecular genetic analysis of dormancy-related traits in poplars. Weed Sci 50:232–240
Chmielewski FM, Rötzer T (2001) Response of tree phenology to climate change across Europe. Agric For Meteorol 108:101–112
Chuine I, Cambon G, Comtois P (2000) Scaling phenology from the local to the regional level: advances from species-specific phenological models. Glob Chang Biol 6:943–952
Colautti RI, Maron JL, Barrett SCH (2009) Common garden comparisons of native and introduced plant populations: latitudinal clines can obscure evolutionary inferences. Evol Appl 2:187–199
Dunlap JM, Stettler RF (1996) Genetic variation and productivity of Populus trichocarpa and its hybrids. IX. Phenology and Melampsora rust incidence of native black cottonwood clones from four river valleys in Washington. For Ecol Manag 87:233–256
Estrella N, Menzel A (2006) Responses of leaf coloring in four deciduous tree species to climate and weather in Germany. Clim Res 32:253–267
Farmer RE (1993) Latitudinal variation in height and phenology of balsam poplar. Silvae Genet 42:148–153
Flint HL (1972) Cold hardiness of twigs of Quercus rubra L. as a function of geographic origin. Ecology 53:1163–1170
Franks SJ, Sim S, Weis AE (2007) Rapid evolution of flowering time by an annual plant in response to a climatic fluctuation. Proc Natl Acad Sci 104:1278–1282
Friedman JM, Auble GT, Shafroth PB, Scott ML, Merigliano MF, Freehling MD, Griffin ER (2005) Dominance of non-native riparian trees in western USA. Biol Invasions 7:747–751
Friedman JM, Roelle JE, Gaskin JF, Pepper AE, Manhart JR (2008) Latitudinal variation in cold hardiness in introduced Tamarix and native Populus. Evol Appl 1:598–607
Gaskin JF, Kazmer DJ (2009) Introgression between invasive saltcedars (Tamarix chinensis and T. ramosissima) in the USA. Biol Invasions 11:1121–1130
Gelman A, Hill J (2007) Data analysis using regression and multilevel/hierarchical models. Cambridge University Press, Cambridge
George MF, Burke MJ, Pellett HM, Johnson AG (1974) Low temperature exotherms and woody plant distribution. Hortscience 9:519–522
Hall D, Luquez V, Garcia VM, St. Onge KR, Jansson S, Ingvarsson PK (2007) Adaptive population differentiation in phenology across a latitudinal gradient in European aspen (Populus tremula, L.): a comparison of neutral markers, candidate genes and phenotypic traits. Evolution 61:2849–2860
Howe GT, Aitken SN, Neale DB, Jermstad KD, Wheeler NC, Chen THH (2003) From genotype to phenotype: unraveling the complexities of cold adaptation in forest trees. Can J Bot 81:1247–1266
Hurka H, Bleeker W, Neuffer B (2003) Evolutionary processes associated with biological invasions in the Brassicaceae. Biol Invasions 5:281–292
Jump AS, Peñuelas J (2005) Running to stand still: adaptation and the response of plants to rapid climate change. Ecol Lett 8:1010–1020
Kaszkurewicz A, Fogg PJ (1967) Growing seasons of cottonwood and sycamore as related to geographic and environmental factors. Ecology 48:785–793
Lesica P, Miles S (2001) Tamarisk growth at the northern margin of its naturalized range in Montana, USA. Wetlands 21:240–246
Linkosalo T, Lechowicz MJ (2006) Twilight far-red treatment advances leaf bud burst of silver birch (Betula pendula). Tree Physiol 26:1249–1256
Luquez V, Hall D, Albrectsen BR, Karlsson J, Ingvarsson P, Jansson S (2008) Natural phenological variation in aspen (Populus tremula): the SwAsp collection. Tree Genet Genomes 4:279–292
Maron JL, Vilà M, Bommarco R, Elmendorf S, Beardsley P (2004) Rapid evolution of an invasive plant. Ecol Monogr 74:261–280
McMillan C (1957) Nature of the plant community. IV. Phenological variation within five woodland communities under controlled temperatures. Am J Bot 44:154–163
Morin X, Augspurger C, Chuine I (2007) Process-based modeling of species’ distributions: what limits temperate tree species’ range boundaries? Ecology 88:2280–2291
Nienstaedt H (1974) Genetic variation in some phenological characteristics of forest trees. In: Lieth H (ed) Phenology and seasonality modeling. Springer-Verlag, New York, pp 389–400
Pauley SS, Perry TO (1954) Ecotypic variation of the photoperiodic response in Populus. J Arnold Arboretum 35:167–188
Perry TO (1971) Dormancy of trees in winter. Science 171:29–36
R Development Core Team (2003) R: a language and environment for statistical computing. http://www.R-project.org. Accessed 02 June 2008
Rehfeldt GE, Ying CC, Spittlehouse DL, Hamilton DA Jr (1999) Genetic responses to climate in Pinus contorta: niche breadth, climate change, and reforestation. Ecol Monogr 69:375–407
Repo T, Zhang G, Ryyppö A, Rikala R, Vuorinen M (2000) The relation between growth cessation and frost hardening in Scots pines of different origins. Trees 14:456–464
Robinson TW (1965) Introduction, spread and aerial extent of saltcedar (Tamarix) in the western states. US Geological Survey Professional Paper 491-A. US Government Printing Office, Washington, DC
Sakai A (1970) Freezing resistance in willows from different climates. Ecology 51:485–491
Sakai A, Larcher W (1987) Frost survival of plants: responses and adaptation to freezing stress. Springer-Verlag, New York
Sakai A, Weiser CJ (1973) Freezing resistance of trees in North America with reference to tree regions. Ecology 54:118–126
Saxe H, Cannell MGR, Johnsen Ø, Ryan MG, Vourlitis G (2001) Tree and forest functioning in response to global warming. New Phytologist 149:369–400
Schaber J, Badeck FW (2003) Physiology-based phenology models for forest tree species in Germany. Int J Biometeorol 47:193–201
Sexton JP, McKay JK, Sala A (2002) Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecol Appl 12:1652–1660
Shafroth PB, Cleverly JR, Dudley TL, Stuart J, Taylor JP, van Riper C, Weeks EP (2005) Control of Tamarix in the western U.S.: implications for water salvage, wildlife use, and riparian restoration. Environ Manag 35:231–246
Sultan SE (2000) Phenotypic plasticity for plant development, function and life history. Trends Plant Sci 5:537–542
Thornton PE, Running SW, White MA (1997) Generating surfaces of daily meteorology variables over large regions of complex terrain. J Hydrol 190:214–251
Tsarouhas V, Kenney WA, Zsuffa L (2001) Variation in freezing resistance during different phenological stages in some Populus and Salix clones: Implications for clonal selection. Silvae Genet 50:54–63
Turesson G (1930) The selective effect of climate upon the plant species. Hereditas 14:99–152
USDA, NRCS (2009) The PLANTS Database. National Plant Data Center, Baton Rouge. http://plants.usda.gov. Accessed 01 June 2009
Van Haverbeke DF (1990) Plains cottonwood. In: Burns RM, Honkala BH (technical coordinators), Silvics of North America: volume 2, Hardwoods. Agriculture Handbook 654, USDA Forest Service, Washington, DC, pp 536–543
Venables WN, Ripley BD (1999) Modern applied statistics with S-Plus, 3rd edn. Springer-Verlag, New York
Viherä-Aarnio A, Häkkinen R, Partanen J, Luomajoki A, Koski V (2005) Effects of seed origin and sowing time on timing of height growth cessation of Betula pendula seedlings. Tree Physiol 25:101–108
Vitasse Y, Delzon S, Dufrêne E, Pontailler JY, Louvet JM, Kremer A, Michalet R (2009) Leaf phenology sensitivity to temperature in European trees: do within-species populations exhibit similar responses? Agric For Meteorol 149:735–744
Vitasse Y, Bresson CC, Kremer A, Michalet R, Delzon S (2010) Quantifying phenological plasticity to temperature in two temperate tree species. Funct Ecol. doi:10.1111/j.1365-2435.2010.01748.x
Wareing PF (1956) Photoperiodism in woody plants. Annual Rev Plant Physiol 7:191–214
Weber E, Schmid B (1998) Latitudinal population differentiation in two species of Solidago (Asteraceae) introduced into Europe. Am J Bot 85:1110–1121
Ying CC, Bagley WT (1976) Genetic variation of eastern cottonwood in an eastern Nebraska provenance study. Silvae Genet 25:67–73
Zhang X, Friedl MA, Schaaf CB, Strahler AH (2004) Climate controls on vegetation phenological patterns in northern mid- and high latitudes inferred from MODIS data. Glob Chang Biol 10:1133–1145
Acknowledgments
Funding was provided by the Global Change Research Program, the Fort Collins Science Center, and the Invasive Species Program of the U.S. Geological Survey. The Common Garden was housed by the Colorado State Forest Service Nursery in Fort Collins. Greenhouse work was carried out at Colorado State University in Fort Collins. J. Roth managed the common garden and collected much of the data. The authors thank G. Auble, L. Benson, and L. Perry and several anonymous referees for constructive reviews. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Online Resource 1
A table showing collection locations and number of individuals planted on 16 August 2005, for plains cottonwood and saltcedar grown in the common garden in Fort Collins, Colorado. (DOCX 13 kb)
Online Resource 2
A figure showing variation in climate among collection locations. (DOCX 48 kb)
Online Resource 3
A table showing mean values of phenological characters by latitude of origin. (DOCX 17 kb)
Online Resource 4
A figure showing overwinter survival in the first year (2005–2006) for plains cottonwood and saltcedar in the common garden as a function of latitude of origin. (DOCX 32 kb)
Rights and permissions
About this article
Cite this article
Friedman, J.M., Roelle, J.E. & Cade, B.S. Genetic and environmental influences on leaf phenology and cold hardiness of native and introduced riparian trees. Int J Biometeorol 55, 775–787 (2011). https://doi.org/10.1007/s00484-011-0494-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-011-0494-6