Abstract
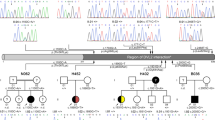
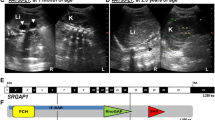
Congenital anomalies of the kidney and urinary tract (CAKUT) can be a part of the VACTERL association, which represents the non-random combination of the following congenital anomalies: vertebral anomalies, anal anomalies, cardiac anomalies, tracheal-esophageal anomalies, kidney anomalies, and limb anomalies. VACTERL association is generally considered to be a non-genetic condition. Exceptions include a patient with a heterozygous nonsense SALL4 variant and anal stenosis, tetralogy of Fallot, sacro-vertebral fusion, and radial and thumb anomalies. SALL4 encodes a transcription factor that plays a critical role in kidney morphogenesis. Here, we report a patient with VACTERL association and a heterozygous 128-kb deletion spanning SALL4 who presented with renal hypoplasia, radial and atrio-septal defects, and patent ductus arteriosus. The present report of SALL4 deletion, in addition to a previously reported patient with VACTERL association phenotype and SALL4 nonsense mutation, further supports the notion that SALL4 haploinsufficiency can lead to VACTERL association.
Similar content being viewed by others
References
Kagan M, Pleniceanu O, Vivante A (2022) The genetic basis of congenital anomalies of the kidney and urinary tract. Pediatr Nephrol 37:2231–2243. https://doi.org/10.1007/s00467-021-05420-1
van de Putte R, Dworschak GC, Brosens E, Reutter HM, Marcelis CLM, Acuna-Hidalgo R, Kurtas NE, Steehouwer M, Dunwoodie SL, Schmiedeke E, Marzheuser S, Schwarzer N, Brooks AS, de Klein A, Sloots CEJ, Tibboel D, Brisighelli G, Morandi A, Bedeschi MF, Bates MD, Levitt MA, Pena A, de Blaauw I, Roeleveld N, Brunner HG, van Rooij I, Hoischen A (2020) A genetics-first approach revealed monogenic disorders in patients with ARM and VACTERL anomalies. Front Pediatr 8:310. https://doi.org/10.3389/fped.2020.00310
Ajam-Hosseini M, Parvini F, Angaji A (2023) A novel de novo nonsense mutation in SALL4 causing duane radial ray syndrome: a case report and expanding the phenotypic spectrum. BMC Med Genom 16:33. https://doi.org/10.1186/s12920-023-01467-1
Borozdin W, Wright MJ, Hennekam RC, Hannibal MC, Crow YJ, Neumann TE, Kohlhase J (2004) Novel mutations in the gene SALL4 provide further evidence for acro-renal-ocular and Okihiro syndromes being allelic entities, and extend the phenotypic spectrum. J Med Genet 41:e102. https://doi.org/10.1136/jmg.2004.019505
Koshiba-Takeuchi K, Takeuchi JK, Arruda EP, Kathiriya IS, Mo R, Hui CC, Srivastava D, Bruneau BG (2006) Cooperative and antagonistic interactions between Sall4 and Tbx5 pattern the mouse limb and heart. Nat Genet 38:175–183. https://doi.org/10.1038/ng1707
Toyoda D, Taguchi A, Chiga M, Ohmori T, Nishinakamura R (2013) Sall4 is transiently expressed in the caudal wolffian duct and the ureteric bud, but dispensable for kidney development. PLoS One 8:e68508. https://doi.org/10.1371/journal.pone.0068508
Wu Q, Chen X, Zhang J, Loh YH, Low TY, Zhang W, Zhang W, Sze SK, Lim B, Ng HH (2006) Sall4 interacts with Nanog and co-occupies Nanog genomic sites in embryonic stem cells. J Biol Chem 281:24090–24094. https://doi.org/10.1074/jbc.C600122200
Guo ZK, Guo K, Luo H, Mu LM, Li Q, Chang YQ (2015) The expression analysis of nanog in the develo** rat myocardial tissues. Cell Physiol Biochem 35:866–874. https://doi.org/10.1159/000369744
Monteon A, Hughes L, Camberos V, Kearns-Jonker M (2023) Identification of SALL4 expressing islet-1+ cardiovascular progenitor cell clones. Int J Mol Sci 24:1780. https://doi.org/10.3390/ijms24021780
Acknowledgements
We would like to thank the patient for permission to publish this article.
Funding
This work was supported by the Japan Agency for Medical Research and Development (grant number JP23ek0109549 and JP23ek0109672) to Kenjiro Kosaki.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Watanabe, D., Nakato, D., Yamada, M. et al. SALL4 deletion and kidney and cardiac defects associated with VACTERL association. Pediatr Nephrol 39, 2347–2349 (2024). https://doi.org/10.1007/s00467-024-06306-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-024-06306-8