Abstract
Background
Children with chronic kidney disease (CKD) require multidisciplinary care to meet their complex healthcare needs. Patient navigators are trained non-medical personnel who assist patients and caregivers to overcome barriers to accessing health services through care coordination. This trial aims to determine the effectiveness of a patient navigator program in children with CKD.
Methods
The NAVKIDS2 trial is a multi-center, waitlisted, randomized controlled trial of patient navigators in children with CKD conducted at five sites across Australia. Children (0–16 years) with CKD from low socioeconomic status rural or remote areas were randomized to an intervention group or a waitlisted control group (to receive intervention after 6 months). The study primary and secondary endpoints include the self-rated health (SRH) (primary), and utility-based quality of life, progression of kidney dysfunction of the child, SRH, and satisfaction with healthcare of the caregiver at 6 months post-randomization.
Results
The trial completed recruitment in October 2021 with expected completion of follow-up by October 2022. There were 162 patients enrolled with 80 and 82 patients randomized to the immediate intervention and waitlisted groups, respectively. Fifty-eight (36%) participants were from regional/remote areas, with a median (IQR) age of 9.5 (5.0, 13.0) years, 46% were of European Australian ethnicity, and 65% were male. A total of 109 children (67%) had CKD stages 1–5, 42 (26%) were transplant recipients, and 11 (7%) were receiving dialysis.
Conclusion
The NAVKIDS2 trial is designed to evaluate the effectiveness of patient navigation in children with CKD from families experiencing socioeconomic disadvantage.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Children and adolescents with chronic kidney disease (CKD) experience an excess risk of death and morbidity. The annual mortality rate for children on dialysis is estimated to be at least thirty times as high as their healthy peers [1]. Children with CKD suffer from chronic pain and discomfort and experience impaired growth and development, frequent hospitalizations, outpatient visits and medical treatments, and reduced quality of life [1, 2]. The effects of this devastating disease also include neurocognitive dysfunction associated with poorer academic achievement, attention dysregulation, impairment of executive functioning [3], and/or physical disabilities that may also be present as part of the phenotype of underlying heritable malformations [4, 5].
The chronicity of this disease and multiple comorbidities require integrated, multidisciplinary care, and treatment plans to meet their complex care needs [6]. Navigating a healthcare system that underpins these complex treatment regimens can be challenging for patients and families [7, 8]. Caregivers of children with CKD are burdened with the responsibility of kee** up with numerous appointments, ensuring adherence to (often multiple) medications, monitoring diet and fluid restrictions, performing time-consuming and involved technical procedures such as home dialysis, and advocating for their child’s health care needs [8]. Consequently, caregivers may experience high levels of physical and psychosocial stress [9,10,11].
In children with CKD, health outcomes are determined not only by the direct effects of the disease but also the complex influences of the social determinants of health and treatment pathways that influence equitable access to care [12,13,14]. Patients from socioeconomically disadvantaged backgrounds are more likely to experience poorer overall health in comparison to their peers from more advantaged backgrounds [14, 15]. Children from remote and rural areas requiring kidney replacement therapy face disparities that can be attributed to barriers to healthcare access, paucity of child-specific resources, and lifelong costs of care [16]. Prior work has shown that children with CKD whose caregivers are unemployed, have lower incomes, poorer self-perceived financial status and lack of home ownership are at least twice as likely to experience poor to fair overall health compared to children with more affluent caregivers [14, 15]. Health inequities in these children are likely to reflect multiple factors including poor access to primary or specialty care, complex health care systems, rigorous and burdensome treatment regimens, lack of awareness of resources, and geographic and language barriers [9,10,11, 17]. The quality of care that these children receive are further influenced by the conflicting priorities and psychosocial stressors faced by the caregivers that can be related to depleting resources caused by continual medical treatment, fatigue and exhaustion from the burden of caring, the demands of tending to family members (e.g., siblings of the patient), and providing financial support to the family [9, 18]. Coordinated patient-centered care that addresses these multi-dimensional factors through a comprehensive delivery of services may be an important tool to support children with CKD and their families [19].
Patient navigators are trained non-medical personnel who assist patients and caregivers with complex and/or chronic conditions to journey through the continuum of care and transit across different care settings. They help vulnerable populations with multi-dimensional care needs to steer through complex health care systems, overcome barriers to health care access, and bridge gaps in transitions of care [20, 21]. A patient navigator program was first developed as a strategy to address barriers to care encountered by cancer patients from a low socioeconomic (SES) background, and to improve cancer education, screening, and timely follow-up. There is evidence in adult patients with chronic disease that suggests patient navigator programs may lead to improved care and outcomes [21]. Patient navigator programs have been trialled with mixed results in adults with CKD. Two randomized controlled trials that assessed the effectiveness of patient navigation to improve access to the deceased donor waiting list reported that potential transplant candidates were twice as likely to complete the number of steps in the evaluation process or were three times as likely to be successfully listed on the deceased donor waiting list if they were assigned to the intervention arm. In contrast, another study noted that patient navigation did not make a difference to the number of steps completed in transplantation work-up and consequently found no difference in the number of patients listed for deceased donor transplant nor a change in the number of living donations. The small sample size and the lack of sufficient power with short follow-up times were some of the limitations identified in these studies [22].
To our knowledge there are no patient navigator studies specifically addressing paediatric nephrology. Among children with chronic conditions, such as diabetes, asthma, and obesity, patient navigator programs are associated with improved access to care, increased medical surveillance, individualized disease management plans, and reduced follow-up visits and hospitalizations [23,24,25,26]. Previous studies have shown that in children with complex care needs, interventions to improve care coordination, such as telehealth and a transition care program for children with solid organ transplants, were effective [27]. The cohorts of patients in these studies included very small numbers of children with kidney transplants. The NAVKIDS2 trial is unique as it is the first and only multi-center randomized waitlisted controlled trial that explores patient navigation in children across all stages of CKD. A holistic approach to the intervention/navigation included provision of education, coordination of health services for the patient, and support directed at the whole family (e.g. caregivers and siblings of the patient). This was not restricted to only medical needs but also general needs of the family, such as organising childcare for siblings, and communicating with the patient’s school [27]. The NAVKIDS2 trial aims to evaluate whether a patient navigator program improves the overall health and well-being of children with CKD who are from low SES backgrounds and/or living in rural/remote areas, compared with standard of care. The primary aim of the study was to compare the self-rated health (SRH) of children with CKD randomized to the immediate intervention arm (patient navigator program) and the waitlisted control arm at 6 months post-randomisation. This paper reports the baseline characteristics of the participants included in the NAVKIDS2 study.
Methods
The NAVKIDS2 trial was conducted at 5 (of 7) paediatric nephrology centres in Australia (Sydney Children’s Hospital at Randwick, The Children’s Hospital at Westmead, The Royal Children’s Hospital in Melbourne, Queensland Children’s Hospital in Brisbane, Perth Children’s Hospital). The design, conduct, and reporting of the study are in accordance with the Consolidated Standards of Reporting Trials [28] (CONSORT) statement as outlined in the published protocol [29]. The trial was registered on the Australia and New Zealand Clinical Trials Registry ACTRN12618001152213.
Eligibility and study population
Children aged 0–16 years (inclusive) with CKD stages 1–5, on dialysis (CKD-D), or with kidney transplants (CKD-T) from a low SES background and/or living in rural or remote areas were eligible to participate in the study. The SES at the time of enrolment was self-reported by the caregivers. Caregivers were asked questions relating to their weekly income, self-perceived SES status, employment status of the primary and secondary caregiver, type of housing (whether living in public housing), and domestic partnership status (refer to Supplement S1). Self-perceived financial status was determined using a 6-point Likert scale of “very poor,” “poor,” “just getting along,” “reasonably comfortable,” “very comfortable,” or “prosperous.” Families were included in the program if the caregivers had a weekly income below the median income of $1250 (AUD) [30], or had a low self-perceived income status, where both partners were unemployed, were living in public housing, or were single parents on social benefits. Families were also eligible if they lived in areas classified as Inner Regional to Very Remote Australia (RA2-RA5). Remote areas included inner regional Australia, outer regional Australia, remote Australia, and very remote Australia [31]. Families were invited to participate in the study if the caregiver(s) either were fluent in English or spoke a little English but had a family member who could communicate in English. This level of communication was sufficient for the navigator assisting participants with day-to-day navigation activities. If required, phone interpreting services were provided during the consenting processes and study visits. The study information sheet was translated to some of the most used languages other than English in Australia (Chinese, Arabic, and Vietnamese languages). Children with a life expectancy of less than 12 months were excluded. Caregivers were also required to give informed consent (and assent if the child was 16 years of age). In families who had more than one child with CKD, only one child could be enrolled in the study to avoid treatment contamination if children in the same family were randomized to different arms. The decision about which sibling to enrol in the study was made by parents/caregivers (as they were likely to have the best understanding of their child’s health and requirement for medical/service). Written consent was only required from one parent/guardian.
Study design
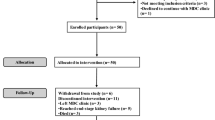
Participants enrolled in the study were randomized with equal probability to the intervention or the waitlisted controlled group, via REDCap [32] research electronic data capture tools hosted at the University of Queensland. Randomization was stratified by CKD stage (CKD 1–5, CKD-D, CKD-T) and site, with random permuted blocks (of varying sizes) used for each stratum. Children in the intervention arm received the patient navigator program immediately after randomization for 6 months. Children in the waitlist control group received standard care during the 6-month “wait-period.” After the wait-period, the control arm received the same patient navigator program for 6 months. Figure 1 shows the study flow.
The intervention
The patient navigator supported patients and caregivers through an individualized plan tailored to the needs of the patients and families to achieve better care and health through involvement in social, community, and health organizational networks. Patient navigators attended a comprehensive blended training program (over a period of 2 weeks) that was developed and delivered by an interdisciplinary team comprised of an experienced patient navigator, social workers, psychologists, consumer partner, data specialist, and members from the NAVKIDS2 trial administration team. All navigators were required to conduct a needs analysis to identify and evaluate/analyze the unmet needs of a family. This enabled the patient navigators to identify issues and prioritize actionable tasks as per the family’s requirement. The domain or categories of tasks derived from the needs analysis included a range of actioning tasks, coordination of services, and identification of support networks. The patient navigator followed a tasks and networks plan [29] that included identification of task categories for a specific patient/family (e.g., following up on prescriptions or referral letters from specialists which patients were awaiting), facilitation (e.g., coordinating communication between caregivers and healthcare professionals), identification of networks (e.g., social support services such as social workers, community-based services, transportation), and documentation and reviewing (e.g., recording time spent on sourcing information for patients).
Navigators provided information on childcare facilities for siblings of patients and coordinated support across multiple services (e.g., physiotherapy, hospital schools, travel assistance schemes for patients living in remote areas, financial support schemes). They helped caregivers and/or patients keep track of appointments, provided educational resources, fostered a more participatory dialogue with clinicians, guided the patients and caregivers to formulate questions relevant to their needs, and advocated for patients. Navigators also provided social and emotional support to caregivers, spending time listening, and empathising with their challenges and burdens.
Outcome measures
The primary study endpoint is the SRH of the child at 6 months post-randomization. SRH is a validated composite measure of global health status [33] and a comprehensive measure that accommodates the World Health Organization (WHO) defined concept of health [34]. This survey technique is commonly adopted for its ease of use and its power in measuring health. As a patient-reported health outcome, SRH is a stable measure of health over time [35] and a statistically powerful predictor of mortality in the general population [36]. SRH has been shown to be inversely correlated with BMI, and with weight status among children and adolescents [37]. In patients with CKD, poor-to-fair SRH is strongly associated with limitations in physical activities of daily living, and adverse events [38]. Studies in the pediatric population have shown that children (from age 8 onwards) can accurately communicate their health symptoms in a meaningful way and can provide valuable insights into their own health [39]. Children below 8 years may have difficulty interpreting and understanding the response categories and a parent proxy measure will be used for children aged 7 years and below. Parent-rated health has been validated for use in young children (as young as 8 weeks old) [40, 41] and is sensitive to chronic conditions and comorbidities in children [37, 42]. The SRH was assessed using the response to a single question “In general, how would you rate your health” and the child was asked to rate their health status on a Likert scale (“5” excellent, “4” very good, “3” good, “2” fair, and “1” poor). For children aged 0–7 years, parent-rated health was used as a proxy measure. The primary outcome analysis is targeted at estimating the difference in SRH of the child between participants randomized to the immediate treatment and waitlisted groups at 6 months post-randomization. All measures of child SRH from baseline to 6 months post-randomization will be analyzed using a cumulative logit mixed effects model, which will include a random intercept for each participant. The primary result will be the treatment effect estimate at 6 months post-randomization and the 95% confidence interval (CI) obtained from the model.
The key secondary endpoints are utility-based quality of life estimates measured using the Health Utilities Index (HUI3) [43], SRH of the child over time, SRH of the caregiver, caregiver satisfaction with healthcare, the number of hospitalizations and missed school days. Other secondary endpoints included direct health-care costs, mortality, and CKD-related outcomes for patients receiving dialysis or post-kidney transplantation (progress of disease dysfunction), up to 12 months post-randomization.
Exploratory endpoints include progression of kidney dysfunction calculated using the CKiD U25 eGFR calculator [44] to calculate the estimated glomerular filtration rate (eGFR), and other biomarkers (urea, albumin, bilirubin, alanine transaminase, alkaline phosphatase, gamma glutamyl transferase, calcium total, phosphate, intact parathyroid hormone, haemoglobin, white cell count, platelets).
Data collection
SRH and HUI3 data were collected from children aged above 8 and 5 years, respectively. The clinical data were cross-checked with medical records. Given the issues with interpreting and responding to questions at a very young age, caregiver-proxy was used for children aged 0–7 years for SRH and 3–5 years for HUI3 or when children were unable or unavailable to complete questionnaires. The HUI3 was not completed for children aged 0–2 years as some of the questions are not relevant to very young children. Although the HUI3 has been used for young children in previous studies [45], it has only been validated for children aged 5 and above; therefore, a sensitivity analysis will be conducted excluding children aged 3–4 years.
Caregiver satisfaction with healthcare was assessed using a survey that asked caregivers about their perceived access to care and confidence in navigating the healthcare system. Progression of kidney dysfunction was calculated for the estimated glomerular filtration rate (eGFR), and other biomarkers (urea, serum creatinine, albumin, bilirubin, alanine transaminase, alkaline phosphatase, gamma glutamyl transferase, calcium, phosphate, intact parathyroid hormone, haemoglobin, white cell count, platelets) were measured from blood samples collected during routine blood tests. The immunosuppressants prescribed were recorded at baseline (time of randomization), and at the end of the treatment for both groups.
Assessments were conducted pre-intervention, 1 month, and 3 months into the intervention, immediately post-intervention, and 6 months post-intervention for the immediate group. For participants who were waitlisted, assessments during the ‘wait-period’ were conducted at baseline, 1 month, and 3 months after randomization and immediately pre-intervention. Assessments, using the same methods as the intervention arm, were conducted 1 month and 3 months into the intervention, and immediately post-intervention (Fig. 1). All study data were collected and managed using REDCap [32] research electronic data capture tools.
Sample size and statistical analysis
The sample size for the trial was calculated for the analysis of the 5-point Likert scale of the SRH of the child (and caregiver-rated health for younger children) using ordinal logistic regression. The target sample size was 150–168 participants, which is 75–84 participants for each arm. This sample size allows the detection of an odds ratio of 2.3 of children from the immediate intervention group reporting higher SRH compared with those in the waitlisted arm with 80% power and a significance level of 5%. It was assumed the dropout rate would be low and so the sample size was not inflated for dropout. For this paper, the baseline characteristics of the study cohort were summarized as counts and percentages for categorical variables, means and standard deviations, and median and interquartile range for continuous variables. The Statistical Analysis System (SAS®) was used to determine the sample size. All data manipulation, tables, figures, listings, and analyses will be documented in SAS®, Stata®, or R programs and performed using SAS version 9.4 or later, Stata version 17 or later, or R version 4.0.0 or later.
Results
Participant recruitment
Recruitment for the NAVKIDS2 trial commenced on July 17, 2020, and was completed on October 20, 2021. In total, 398 participants were screened, with 80 participants randomized to the immediate group and 82 to the waitlist group (Fig. 2). 63% of those who were eligible for study inclusion were randomized. Table 1 provides details of the recruitment by site.
Demographics of the participants
The baseline characteristics of the participants enrolled in NAVKIDS2 are shown in Table 1. Most of the participants (64%) were from metropolitan areas, almost a third of the participants (31%) were from inner or outer regional Australia, while the rest (4%) were from remote or very remote areas. The largest proportion of participants were of European Australian (46%) ethnicity, while people of Middle Eastern (9%), Asian (14%) ethnicities and those identifying as Aboriginal, or Torres Strait Islander people (9%) and Pacific Islander people (3%) together comprised over a third of the cohort. Most of the children (95%) spoke English at home, while 17% children spoke a second language other than English at home. The median age of the children was 9.5 years (interquartile range (IQR) (5.0, 13.0) years) with an overall predominance of males (65%) enrolled in the study.
Characteristics of the family unit
Table 2 provides details on the families enrolled in the study. Over 68% of the children belonged to households earning less than the Australian median weekly income of $1250, with almost 30% of the families earning less than $600 per week. While 15% of the caregivers considered themselves as poor or very poor, 117 (72%) of the caregivers felt they were “just getting along.” A breakdown of participants by SES and rurality showed that approximately 5% of the participants were included in the study because they were from rural or remote areas while 64% were included in the study because they met the SES criteria. Of note, 31% of the participants met the remoteness and the SES criteria.
Approximately one-fifth (22%) of the children came from backgrounds where both of their caregivers were unemployed, 28% had a single caregiver on social benefits, and 11% lived in public housing. Over half of the primary caregivers were unemployed while a third either had part time, casual, or contract jobs (Table S2.1). Only 17% of the primary caregivers held full time jobs. However, in the case of the secondary caregivers (Table S2.2), 51% were employed full time, a quarter were unemployed, while the rest held part time, casual, or contract jobs.
All except two children were living with their primary caregivers at enrolment, with the mother being the primary caregiver of 85% of the children (Table S2.1). A small proportion of the children had fathers (9%) or grandparents (5%) as primary caregivers. The mean age (standard deviation (SD)) of the primary caregivers was 39 (8.8) years and 31% held a bachelor’s degree or higher. A third of the primary caregivers listed their highest level of education as secondary school, while 36% reported primary school, diploma/other certificate, or trade certificate as the highest level of education (Table S2.1).
Clinical history of the participants
The median (IQR) serum creatinine levels, estimated glomerular filtration rate (eGFR), and body mass index (BMI) Z-score at baseline were 73.5 (44.0, 142.8) µmol/L, 61.9 (35.0, 101.1) mL/min/1.73 m2, and 0.7 (− 0.5, 1.3), respectively (Table 1). Details of the children’s clinical history and cause of CKD are shown in Table 3. Neurodevelopmental conditions and hypertension were the most common conditions with each being reported by over one fifth of the children, while growth deficiency, chronic infections, cardiovascular, and gastrointestinal conditions were each reported by 10–15% of the children. Over 5% of the children had bone disease, endocrine, hearing, respiratory, or urological conditions. A small proportion of children (under 4%) reported diabetes, hematological, cancer, or mental health and behavioral conditions. The most common causes of CKD were congenital anomalies of kidney and urinary tract (CAKUT) (n = 74, 46%), followed by glomerular disease (n = 45, 28%), and 19% of the children had kidney disease of unknown/other origin. A small number of children had cystic kidney disease (n = 13, 8%). Sixty seven percent (n = 109) of the children had CKD stage 1–5 at baseline, while over a quarter (n = 42, 26%) of the cohort were classified as CKD-T. A total of 11 children were treated with dialysis with 3 children on hemodialysis and 8 on peritoneal dialysis. Of the 11 children on dialysis, 3 children had a potential live donor, while 6 children were on a deceased donor waiting list.
Over a third (n = 60, 37%) of the children were treated with immunosuppression, with tacrolimus (90%) and prednisone (80%) being the most common immunosuppressive therapies reported by the group. Almost two-thirds (65%) of the children on immunosuppressants were prescribed mycophenolate, while less than 10% of children on immunosuppressants were treated with azathioprine or everolimus (Table S2.3). Approximately a quarter (24%) of the children were on 3 immunosuppressants, while a small number of children (6% or 7%) were prescribed 1 or 2 immunosuppressants, respectively. Some children (4%) were prescribed over 10 concomitant medicines. The median (IQR) number of prescribed concomitant medicines was 3 (1,5) (Table S2.4).
Discussion
The NAVKIDS2 study is an ongoing multi-center randomized controlled trial designed to examine the effectiveness of patient navigation in children with CKD from low SES backgrounds or living in rural and remote areas. The baseline characteristics of the study participants are consistent with the goal to recruit children from families of low SES backgrounds and/or living in rural/remote Australia.
The CKD stage of included participants is consistent with the Kidney Disease: Improving Global Outcomes (KDIGO) [46] classification of children with kidney disease. In the NAVKIDS2 trial, approximately 26% of the included participants were kidney transplant recipients, 7% were treated with maintenance dialysis, and 67% had CKD stages 1–5 at baseline. The children also had complex comorbid conditions including chronic infections, growth deficiency, cardiovascular, and gastrointestinal disorders, which require care coordination between multidisciplinary teams.
The NAVKIDS2 trial will be the first randomized trial to report the clinical and quality of life outcomes of a patient navigation program in children across the full spectrum of CKD. The trial is expected to provide insights into the impact of a patient navigation program on overall health and wellbeing of children with CKD who are experiencing social disadvantage. This trial is also expected to identify the challenges, barriers, and enablers that families from low SES and/or from rural and remote areas encounter while navigating through the complex healthcare system. Information gathered through various sources including qualitative semi-structured interviews with patient families, navigators, and health care providers will provide an in-depth understanding of the role of a navigator in enabling families to transit across multiple care settings and of the implementation and sustainability of a navigator program.
Prior randomized controlled trials have explored the effectiveness of patient navigation in other settings including transition programs from pediatrics to adult care, education, disease management, and psychosocial support across a variety of chronic conditions [47,48,49]. Previous studies [50, 51] have shown that care coordination may improve disease management behaviors and reduce morbidity in children with asthma. Coordination of care included establishment of individualized asthma management goals, improving health literacy, facilitation of interactions with health care system, and building social support for improved asthma management. Studies have also provided evidence that parents of infants and children who have been hospitalized or been newly diagnosed with a chronic condition may find the support of care coordinators (for example a nurse or a peer navigator who could be a parent) beneficial [52, 53]. These studies have shown that the provision of parental support may improve parental mental health and family functioning. A systematic review [47] of transition interventions for adolescents with chronic illness has discussed studies on transitional programs involving case managers and coordinators that showed higher rates of transfer of care in the transition program participants.
The NAVKIDS2 trial is a unique study that addresses patient navigation in children with CKD. Navigation in this trial is not only designed for specific health needs of the patients but also addresses the unmet needs of the family members including the siblings and the caregivers/parents of the patient. The role of the patient navigator in this trial includes working with the family to understand their needs, develo** an individualized plan to coordinate support towards the fulfillment of these needs, real-time assistance with navigating the healthcare and social services systems, reducing family stress, and breaking down community barriers. These services are not only designed to meet the healthcare needs of the children but also extend to the broader social and economic needs of the entire family unit, in accordance with the focus on impacts of social determinants of health. Specifically, the trial will address the unmet needs of the families from low socioeconomic background and/or remote/rural areas and ensure equitable access to healthcare. The trial also seeks to explore the feasibility and viability of the implementation and sustainability of such a program across all stages of pediatric CKD. Costs directly related to the trial, the intended and unintended downstream effects of the intervention will be assessed, and the cost-effectiveness calculated [29].
The COVID-19 pandemic has imposed challenges on the NAVKIDS2 trial and affected trial activities including recruiting, consenting, data collection, and management. Recruitment was paused during lockdown, and face-to-face access to patients was limited to prioritize patient safety. Mitigations were put in place to ensure continuity of trial activities such as recruitment and informed consent which were conducted verbally via phone. However, despite these restrictions, recruitment reached the revised target. Trial processes were adjusted to ensure the continuum of the trial activities; e.g., patient navigators had flexible arrangements and the option to carry out face-to-face or virtual consultations with the families.
Conclusion
In summary, it is expected that this trial will provide high certainty evidence on the effectiveness of a patient navigation program for children with CKD from low SES backgrounds and/or rural/remote areas. It will also provide insights into adaptations required in different settings, sustainability, continuity, and dissemination of this program, if proven successful, in clinical settings. This trial will reveal information on how patient preferences and shared decisions can be integrated in healthcare to ultimately improve health outcomes in those children with CKD and families that experience the greatest disparity.
Availability of data and material
The dataset generated during and/or analyzed during the current study are available from corresponding author on reasonable request, beginning 2 years following main publication.
References
McDonald SP, Craig JC (2004) Long-term survival of children with end-stage renal disease. N Engl J Med 350:2654–2662. https://doi.org/10.1056/NEJMoa031643
Baum M (2010) Overview of chronic kidney disease in children. Curr Opin Pediatr 22:158–160. https://doi.org/10.1097/MOP.0b013e32833695cb
Wong CJ, Moxey-Mims M, Jerry-Fluker J, Warady BA, Furth SL (2012) CKiD (CKD in children) prospective cohort study: a review of current findings. Am J Kidney Dis 60:1002–1011. https://doi.org/10.1053/j.ajkd.2012.07.018
Salomon R, Saunier S, Niaudet P (2009) Nephronophthisis. Pediatr Nephrol 24:2333–2344. https://doi.org/10.1007/s00467-008-0840-z
Ahn SY, Mendoza S, Kaplan G, Reznik V (2009) Chronic kidney disease in the VACTERL association: clinical course and outcome. Pediatr Nephrol 24:1047–1053. https://doi.org/10.1007/s00467-008-1101-x
Ajarmeh S, Er L, Brin G, Djurdjev O, Dionne JM (2012) The effect of a multidisciplinary care clinic on the outcomes in pediatric chronic kidney disease. Pediatr Nephrol 27:1921–1927. https://doi.org/10.1007/s00467-012-2209-6
Blydt-Hansen TD, Pierce CB, Cai Y, Samsonov D, Massengill S, Moxey-Mims M, Warady BA, Furth SL (2014) Medication treatment complexity and adherence in children with CKD. Clin J Am Soc Nephrol 9:247–254. https://doi.org/10.2215/cjn.05750513
Tong A, Sainsbury P, Chadban S, Walker RG, Harris DC, Carter SM, Hall B, Hawley C, Craig JC (2009) Patients’ experiences and perspectives of living with CKD. Am J Kidney Dis 53:689–700. https://doi.org/10.1053/j.ajkd.2008.10.050
Medway M, Tong A, Craig JC, Kim S, Mackie F, McTaggart S, Walker A, Wong G (2015) Parental perspectives on the financial impact of caring for a child With CKD. Am J Kidney Dis 65:384–393. https://doi.org/10.1053/j.ajkd.2014.07.019
Mahmoud DAM, Saad A, Abdelhamid YH, El Hawary Y (2021) Depression and psychosocial burden among caregivers of children with chronic kidney disease. Middle East Curr Psychiatry 28:12. https://doi.org/10.1186/s43045-021-00092-x
Geense WW, van Gaal BGI, Knoll JL, Cornelissen EAM, van Achterberg T (2017) The support needs of parents having a child with a chronic kidney disease: a focus group study. Child Care Health Dev 43:831–838. https://doi.org/10.1111/cch.12476
Kaspar CDW, Bholah R, Bunchman TE (2016) A review of pediatric chronic kidney disease. Blood Purif 41:211–217. https://doi.org/10.1159/000441737
Minnick ML, Boynton S, Ndirangu J, Furth S (2010) Sex, race, and socioeconomic disparities in kidney disease in children. Semin Nephrol 30:26–32
Didsbury MS, Kim S, Medway MM, Tong A, McTaggart SJ, Walker AM, White S, Mackie FE, Kara T, Craig JC, Wong G (2016) Socio-economic status and quality of life in children with chronic disease: a systematic review. J Paediatr Child Health 52:1062–1069
Didsbury M, van Zwieten A, Chen K, James LJ, Francis A, Kim S, McTaggart S, Walker A, Mackie F, Kara T, Prestidge C, Teixeira-Pinto A, Barton B, Lorenzo J, Lah S, Howard K, Nassar N, Au E, Tong A, Craig JC, Wong G (2019) The association between socioeconomic disadvantage and parent-rated health in children and adolescents with chronic kidney disease—the Kids with CKD (KCAD) study. Pediatr Nephrol 34:1237–1245. https://doi.org/10.1007/s00467-019-04209-7
Lalji R, Francis A, Wong G, Viecelli AK, Tong A, Teixeira-Pinto A, McCulloch M, Bello AK, Levin A, Lunney M, Osman MA, Ye F, Jha V, Feehally J, Harris DC, Johnson DW (2020) Disparities in end-stage kidney disease care for children: a global survey. Kidney Int 98:527–532. https://doi.org/10.1016/j.kint.2020.04.058
Coombes J, Hunter K, Mackean T, Holland AJA, Sullivan E, Ivers R (2018) Factors that impact access to ongoing health care for First Nation children with a chronic condition. BMC Health Serv Res 18:448. https://doi.org/10.1186/s12913-018-3263-y
Friedman AL (2006) The broader burden of end-stage renal disease on children and their families. Kidney Int 70:1893–1894. https://doi.org/10.1038/sj.ki.5001964
Kodner DL, Spreeuwenberg C (2002) Integrated care: meaning, logic, applications, and implications–a discussion paper. Int J Integr Care 2:e12–e12. https://doi.org/10.5334/ijic.67
Natale-Pereira A, Enard KR, Nevarez L, Jones LA (2011) The role of patient navigators in eliminating health disparities. Cancer 117(15 Suppl):3543–3552. https://doi.org/10.1002/cncr.26264
McBrien KA, Ivers N, Barnieh L, Bailey JJ, Lorenzetti DL, Nicholas D, Tonelli M, Hemmelgarn B, Lewanczuk R, Edwards A, Braun T, Manns B (2018) Patient navigators for people with chronic disease: a systematic review. PLoS One 13:e0191980–e0191980. https://doi.org/10.1371/journal.pone.0191980
Cervantes L, Hasnain-Wynia R, Steiner JF, Chonchol M, Fischer S (2020) Patient navigation: addressing social challenges in dialysis patients. Am J Kidney Dis 76:121–129. https://doi.org/10.1053/j.ajkd.2019.06.007
Coughey K, Klein G, West C, Diamond JJ, Santana A, McCarville E, Rosenthal MP (2010) The Child Asthma Link Line: a coalition-initiated, telephone-based, care coordination intervention for childhood asthma. J Asthma 47:303–309. https://doi.org/10.3109/02770900903580835
Van Walleghem N, MacDonald CA, Dean HJ (2008) Evaluation of a systems navigator model for transition from pediatric to adult care for young adults with type 1 diabetes. Diabetes Care 31:1529–1530. https://doi.org/10.2337/dc07-2247
Black HL, Priolo C, Akinyemi D, Gonzalez R, Jackson DS, Garcia L, George M, Apter AJ (2010) Clearing clinical barriers: enhancing social support using a patient navigator for asthma care. J Asthma 47:913–919. https://doi.org/10.3109/02770903.2010.506681
Knierim SD, Moore SL, Raghunath SG, Yun L, Boles RE, Davidson AJ (2018) Home visitations for delivering an early childhood obesity intervention in Denver: parent and patient navigator perspectives. Matern Child Health J 22:1589–1597. https://doi.org/10.1007/s10995-018-2553-7
Lalji R, FrancisA KR, Guha C, Johnson DW, Wong G (2021) Patient navigator programmes for children and adolescents with chronic diseases. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD014688
Schulz KF, Altman DG, Moher D, CONSORT Group (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 152:726–732. https://doi.org/10.7326/0003-4819-152-11-201006010-00232%m20335313
van Zwieten A, Caldwell P, Howard K, Tong A, Craig JC, Alexander S, Howell M, Armando TP, Hawley C, Jesudason S, Walker A, Mackie F, Kennedy S, McTaggart S, McCarthy H, Carter S, Kim S, Crafter S, Woodleigh R, Guha C, Wong G (2019) NAV-KIDS 2 trial: protocol for a multi-centre, staggered randomised controlled trial of a patient navigator intervention in children with chronic kidney disease. BMC Nephrol 20:134
Australian Bureau of Statistics, Household income and wealth, Australia. 2019–2020 12/05/2022]; Available from: https://www.abs.gov.au/statistics/economy/finance/household-income-and-wealth-australia/latest-release#media-releases. Accessed 16 Sept 2022
Australian Government, Department of Health and Aged care, Australian statistical geography standard — remoteness area. 14 December 2021 [cited 2022 21 January]; Available from: https://www.health.gov.au/health-topics/rural-health-workforce/classifications/asgs-ra#how-we-use-it. Accessed 16 Sept 2022
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. https://doi.org/10.1016/j.jbi.2008.08.010
Schnittker J, Bacak V (2014) The increasing predictive validity of self-rated health. PLoS One 9:e84933. https://doi.org/10.1371/journal.pone.0084933
Bowling A (2005) Just one question: If one question works, why ask several? J Epidemiol Community Health 59:342. https://doi.org/10.1136/jech.2004.021204
Fosse NE, Haas SA (2009) Validity and stability of self-reported health among adolescents in a longitudinal, nationally representative survey. Pediatrics 123:e496-501. https://doi.org/10.1542/peds.2008-1552
Idler EL, Benyamini Y (1997) Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav 38:21–37
Herman KM, Sabiston CM, Tremblay A, Paradis G (2014) Self-rated health in children at risk for obesity: associations of physical activity, sedentary behaviour, and BMI. J Phys Act Health 11:543–552. https://doi.org/10.1123/jpah.2012-0124
Lee J, Abdel-Kader K, Yabes JG, Cai M, Chang HH, Jhamb M (2021) Association of self-rated health with functional limitations in patients with CKD. Kidney Med 3:745-752.e1. https://doi.org/10.1016/j.xkme.2021.04.010
Riley AW (2004) Evidence that school-age children can self-report on their health. Ambul Pediatr 4(4 Suppl):371–376. https://doi.org/10.1367/a03-178r.1
Shrivastava A, Murrin C, Kelleher CC (2014) Preschoolers’ parent-rated health disparities are strongly associated with measures of adiposity in the Lifeways cohort study children. BMJ Open 4:e005328. https://doi.org/10.1136/bmjopen-2014-005328
Spencer NJ, Coe C (2000) Validation of the Warwick Child Health and Morbidity profile in routine child health surveillance. Child Care Health Dev 26:323–336. https://doi.org/10.1046/j.1365-2214.2000.00148.x
Schnittker J, Bacak V (2014) The increasing predictive validity of self-rated health. PLoS One 9:e84933–e84933. https://doi.org/10.1371/journal.pone.0084933
Horsman J, Furlong W, Feeny D, Torrance G (2003) The Health Utilities Index (HUI®): concepts, measurement properties and applications. Health Qual Life Outcomes 1:54. https://doi.org/10.1186/1477-7525-1-54
Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ (2021) Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int 99:948–956. https://doi.org/10.1016/j.kint.2020.10.047
Rowen D, Keetharuth AD, Poku E, Wong R, Pennington B, Wailoo A (2021) A review of the psychometric performance of selected child and adolescent preference-based measures used to produce utilities for child and adolescent health. Value Health 24:443–460. https://doi.org/10.1016/j.jval.2020.09.012
Kidney Disease: Improving Global Outcomes (KDIGO) CKD work group (2013) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013:1–150
Chu PY, Maslow GR, von Isenburg M, Chung RJ (2015) Systematic review of the impact of transition interventions for adolescents with chronic illness on transfer from pediatric to adult healthcare. J Pediatr Nurs 30:e19–e27. https://doi.org/10.1016/j.pedn.2015.05.022
Krieger JW, Takaro TK, Song L, Weaver M (2005) The Seattle-King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health 95:652–659. https://doi.org/10.2105/AJPH.2004.042994
Raphael JL, Rueda A, Lion KC, Giordano TP (2013) The role of lay health workers in pediatric chronic disease: a systematic review. Acad Pediatr 13:408–420. https://doi.org/10.1016/j.acap.2013.04.015
Findley S, Rosenthal M, Bryant-Stephens T, Damitz M, Lara M, Mansfield C, Matiz A, Nourani V, Peretz P, Persky VW, Valencia GR, Uyeda K, Viswanathan M (2011) Community-based care coordination: practical applications for childhood asthma. Health Promot Pract 12(6_suppl_1):52S-62S. https://doi.org/10.1177/1524839911404231
Seid M, Varni JW, Gidwani P, Gelhard LR, Slymen DJ (2010) Problem-solving skills training for vulnerable families of children with persistent asthma: report of a randomized trial on health-related quality of life outcomes. J Pediatr Psychol 35:1133–1143. https://doi.org/10.1093/jpepsy/jsp133
Sullivan-Bolyai S, Grey M, Deatrick J, Gruppuso P, Giraitis P, Tamborlane W (2004) Hel** other mothers effectively work at raising young children with type 1 diabetes. Diabetes Educ 30:476–484. https://doi.org/10.1177/014572170403000319
Kauffmann E, Harrison MB, Burke SO, Wong C (1998) Stress-point intervention for parents of children hospitalized with chronic conditions. Pediatr Nurs 24:362–366
Acknowledgements
Trial Steering Committee:
Germaine Wong (Co-chair), Centre for Kidney Research at The Children’s Hospital at Westmead and Sydney School of Public Health, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia; Carmel Hawley (Co-Chair), Princess Alexandra Hospital, the Translational Research Institute and Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Allison Tong, Sydney School of Public Health, The University of Sydney; Amanda Walker, Department of Nephrology, Royal Children’s Hospital, Melbourne, Australia; Amelie Bernier-Jean, University of Montreal, Canada; Anita van Zwieten, Centre for Kidney Research at The Children’s Hospital at Westmead and Sydney School of Public Health, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia; Anna Francis, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia; Armando Teixeira-Pinto, Centre for Kidney Research at The Children’s Hospital at Westmead and Sydney School of Public Health, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia; Alistair Mallard, Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Chandana Guha, Centre for Kidney Research at The Children’s Hospital at Westmead and Sydney School of Public Health, Faculty of Medicine and Health, The University of Sydney, Sydney Australia; Charani Kiriwandeniya, Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; David Johnson, Princess Alexandra Hospital, the Translational Research Institute and Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Deirdre Hahn, Centre for Kidney Research at The Children’s Hospital at Westmead, Sydney, Australia; Donna Reidlinger, Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Elaine Pascoe, Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Elizabeth Ryan, QCIF Facility for Advanced Bioinformatics and Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Fiona Mackie, Department of Renal Medicine, Sydney Children’s Hospital, Randwick, Sydney, Australia; Hugh J McCarthy, Centre for Kidney Research at The Children’s Hospital at Westmead and Department of Renal Medicine, Sydney Children’s Hospital, Randwick, Sydney, Australia; Jonathan Craig, College of Medicine and Public Health, Flinders University, Adelaide, Australia; Julie Varghese, Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Kirsten Howard, Sydney School of Public Health, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia; Liza Vergara, Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Luke Macauley, The University of Sydney, Sydney, Australia; Martin Howell, Sydney School of Public Health, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia; Michelle Irving, The University of Sydney, Sydney Australia; Nicholas Larkins, Perth Children’s Hospital, Perth, Australia; Patrina Caldwell, Centre for Kidney Research at The Children’s Hospital at Westmead and Discipline of Child and Adolescent Health, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia; Rabia Khalid, Centre for Kidney Research at The Children’s Hospital at Westmead, Sydney, Australia; Reg Woodleigh, Prostate and Breast Cancer Foundation (CanCare), Sydney, Australia; Sean Kennedy, Department of Renal Medicine, Sydney Children’s Hospital, Randwick, Sydney, Australia; Shilpanjali Jesudason, Department of Renal Medicine, Royal Adelaide Hospital, Adelaide, Australia; Simon Carter, Department of Nephrology, Royal Children’s Hospital, Melbourne, Australia; Stephen Alexander, Centre for Kidney Research at The Children’s Hospital at Westmead, Sydney, Australia; Steve McTaggart, Children’s Health Queensland Hospital and Health Service and The University of Queensland, Australia
Safety Monitoring Committee:
Jennifer Couper (Chair), Women’s and Children’s Hospital Adelaide and University of Adelaide, Adelaide, Australia; Thomas Snelling (Former Chair), Sydney School of Public Health, University of Sydney and Telethon Kids Institute, Perth, Australia; David Issacs, Sydney Children’s Hospital Network, Sydney, Australia; Megan Cann, Children’s Health Queensland Hospital and Health Service, Brisbane, Australia; Ushma Wadia, Perth Children’s Hospital and Telethon Kids Institute, Perth, Australia
Collaborating sites and investigators:
The Children’s Hospital Westmead (Germaine Wong, Hugh J McCarthy, Stephen Alexander, Deirdre Hahn, Siah Kim, Anne Durkan, Gayathri Raman, Blake Sandery, Rabia Khalid, Chandana Guha), Sydney Children’s Hospital (Sean Kennedy, Hugh McCarthy, Fiona Mackay, Suzanne Nevin, Amy Dalton, Rabia Khalid), Queensland Children’s Hospital (Anna Francis, Steve McTaggart, Rowena Lalji, Kali Juracek, Sophie Anderson-James), Perth Children’s Hospital (Nicholas Larkins, Kristy Nicholl), Royal Children’s Hospital (Amanda Walker, Simon Carter, Madeleine Didsbury, Brendan Cusack)
Project management team:
The Australasian Kidney Trials Network, Brisbane, Australia: Carmel Hawley, David Johnson, Donna Reidlinger, Elaine Pascoe, Alistair Mallard, Liza Vergara, Charani Kiriwandeniya, Elizabeth Ryan, Julie Varghese
Centre for Kidney Research, Sydney, Australia: Germaine Wong, Anita van Zwieten, Rabia Khalid, Chandana Guha
Australasian Kidney Trials Network (AKTN) Scientific Committee:
Carmel Hawley, Department of Nephrology, Princess Alexandra Hospital, Woolloongabba, Australia; the Translational Research Institute and Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Jessica Dawson, NHMRC Clinical Trial Centre, University of Sydney, NSW, Australia, and St George Hospital, Kogarah, NSW, Australia; Rachael Walker, Hawke’s Bay District Health Board and Eastern Institute of Technology, The University of Sydney, Sydney, Australia; Elaine Pascoe, Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Nicholas Larkins, Perth Children’s Hospital, Perth, Australia; Sunil V. Badve, Department of Renal Medicine, St George Hospital, Sydney, Australia, and Renal and Metabolic Division, The George Institute for Global Health, University of New South Wales Medicine, Sydney, Australia; Neil Boudville, Sir Charles Gairdner Hospital, Perth, Australia, and Division of Internal Medicine, Medical School, University of Western Australia, Perth, Australia; Meg J. Jardine, NHMRC Clinical Trials Centre, Faculty of Medicine and Health, University of Sydney, Australia; Concord Repatriation and General Hospital, Concord, Australia; and The George Institute for Global Health, University of New South Wales, Sydney, Australia; Yeoungjee Cho, Department of Nephrology, Princess Alexandra Hospital, Woolloongabba, Australia, and Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Rathika Krishnasamy, Sunshine Coast University Hospital, Australia, and Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Vlado Perkovic, The George Institute for Global Health, University of New South Wales, Sydney, Australia; Magid A. Fahim, Department of Nephrology, Princess Alexandra Hospital, Woolloongabba, Australia, and Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Matthew A. Roberts, Eastern Health Clinical School, Monash University, Melbourne, Australia; Michael Collins, Auckland City Hospital and University of Auckland, Auckland, New Zealand; Donna Reidlinger, Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Laura Robison, Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Fabian Marsden, AKTN Scientific Committee.
AKTN Executive Operations Secretariat:
Carmel Hawley (Chair), Department of Nephrology, Princess Alexandra Hospital, Woolloongabba, Australia; the Translational Research Institute and Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; David Johnson (Deputy Chair), Department of Nephrology, Princess Alexandra Hospital, Woolloongabba, Australia; the Translational Research Institute and Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; and Translational Research Institute, Brisbane, Australia; Matthew Roberts, Eastern Health Clinical School, Monash University, Melbourne, Australia; Michael Collins, Auckland City Hospital and University of Auckland, Auckland, New Zealand; Neil Boudville, Sir Charles Gairdner Hospital, Perth, Australia and Division of Internal Medicine, Medical School, University of Western Australia, Perth, Australia; Meg J. Jardine, NHMRC Clinical Trials Centre, Faculty of Medicine and Health, University of Sydney, Australia; Concord Repatriation and General Hospital, Concord, Australia; and The George Institute for Global Health, University of New South Wales, Sydney, Australia; Magid A. Fahim, Department of Nephrology, Princess Alexandra Hospital, Woolloongabba, Australia, and Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Yeoungjee Cho, Department of Nephrology, Princess Alexandra Hospital, Woolloongabba, Australia, and Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Rathika Krishnasamy, Sunshine Coast University Hospital, Australia, and Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Donna Reidlinger, Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Elaine Pascoe, Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Laura Robison, Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Sunil V. Badve, Department of Renal Medicine, St George Hospital, Sydney, Australia, and Renal and Metabolic Division, the George Institute for Global Health, University of New South Wales Medicine, Sydney, Australia; Andrea Viecelli, Department of Nephrology, Princess Alexandra Hospital, Woolloongabba, Australia and Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Liza Vergara, Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; Vlado Perkovic, The George Institute for Global Health, University of New South Wales, Sydney, Australia; Alistair Mallard, Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia.
Other acknowledgments:
We would like to acknowledge all our patient navigators for their contributions to the study.
REDCap [32] UQ: Study data were collected and managed using the REDCap [32] electronic data capture tools hosted at the University of Queensland. REDCap [32] (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for data integration and interoperability with external sources.
Funding
The study is funded by a National Health and Medical Research Council fund under the category: Medical Research Future Fund Targeted Call for Research: Rare Cancers, Rare Diseases and Unmet Need Initiative (APP1170021).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Chandana Guha, Rabia Khalid, and Elizabeth Ryan. The first draft of the manuscript was written by Chandana Guha, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics declaration
Ethics approval for NAVKIDS2 study was obtained from Sydney Children’s Hospital Network Human Research Ethics Committee (approval HREC/18/SCHN/325) and the University of Queensland Human Research Ethics Committee (clearance number 2019002528).
Consent to participate
Consent has been obtained from parents of all the participants and assent from participants aged 16 or of sufficient maturity in the NAVKIDS2 trial.
Consent for publication
Not applicable.
Conflict of interest
RW is the Program Director of CanCare, a patient navigation program for cancer patients run by the Prostate and Breast Cancer Foundation, Australia. DJ has received consultancy fees, research grants, speaker’s honoraria, and travel sponsorships from Baxter Healthcare and Fresenius Medical Care; consultancy fees from Astra Zeneca, Bayer, and AWAK; speaker’s honoraria from ONO and BI & Lilly; and travel sponsorships from Ono and Amgen. He is a current recipient of an Australian National Health and Medical Research Council Leadership Investigator Grant. CH institution received Baxter (provision of investigational agent for a study). Other authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guha, C., Khalid, R., van Zwieten, A. et al. Baseline characteristics of participants in the NAVKIDS2 trial: a patient navigator program in children with chronic kidney disease. Pediatr Nephrol 38, 1577–1590 (2023). https://doi.org/10.1007/s00467-022-05772-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05772-2